Accelerating Processes
Laboratory of the Future: Digitally Connected and Automated
Laboratories reap the full benefits of automation and data science when they prioritise digitalisation by implementing the right digital tools
Ian Yates at Thermo Fisher Scientific
To meet the ever-growing demands in drug development and manufacturing, teams are often required to accelerate operations, while maintaining data accuracy and regulatory compliance. Given the shift to capturing multiple data modalities within a single project, the scientific ecosystem is experiencing a ‘new normal’ as Big Data slowly become the currency of scientific capability. Generating copious amounts of data is, however, only one side of the story. Equally important are the systems used to manage them.
While most companies appreciate the importance of automated workflows in boosting throughput and efficiency, digital connectivity can be overlooked. Although digitalisation is expected to continue to disrupt the pharmaceutical industry in the next five years, most organisations feel unprepared (1). The COVID-19 pandemic, however, has played a part in compelling pharma companies to prioritise digitalisation due to the critical need to enable remote working and maintain supply chain security to ensure on-time product release.
Here, we discuss how laboratories can integrate automated instruments with digital systems to achieve end-to-end digital connectivity that fosters data integrity and companywide collaboration.
Lab-Specific Automation Considerations
When labs start considering automation, three common themes emerge: user acceptance, physical constraints, and digital capabilities. Of utmost importance is the scientists’ experience as it relates to automating tasks, for example, replacing manual pipetting with liquid handlers. Automated tools such as these can boost sample throughput, maintain data accuracy, and improve reproducibility, especially during challenging workflows. However, when implementing these solutions, it’s key to also consider the scientist’s role in the workflow. With hands-on technical knowledge, and years of optimisation and troubleshooting experience, scientists know the minutiae of every protocol, and can promptly gauge if a particular automated solution is the right choice.
To ensure good user experience with automation, labs can consider the following:
- Can automation increase lab output with minimal supervision, while still providing reliable results?
- How intuitive or user-friendly is the interface?
- How flexible or versatile are the instruments for multiple applications?
With regard to physical constraints, the amount of lab space available often restricts the type of automation that can be considered. Bringing sizeable equipment into the limited physical space of a functioning lab can obstruct existing workflows. As such, modern, modular, spacesaving automation solutions are often preferred. Moreover, a system’s compatibility with existing instruments will influence its acceptance rate: the more seamless the workflow, the more positive an impact it will have on scientists’ work.
To enable a smooth transition to automation, consider the following lab- and workflow-related factors:
- Can the automation system be configured to accommodate the current lab layout?
- How many tasks or workflows can we automate with one system? Are there bottlenecks in the workflow?
- As workflows evolve, how easy is it to update the automation system with minimal interruption?
Finally, the true potential of automation is only realised when labs implement the right digital tools to manage data and processes. Fundamentally, digitalisation is an essential link between automation and advanced data analytics, empowering companies to accelerate their current operations, as well as reap the benefits of data-driven science in the near future.
Achieving Digital Transformation in Three Steps
Automation offers as much of a practical solution to address lab throughput and efficiency as it provides a strategic upper hand by collating large volumes of highquality datasets into one centralised location, substantially simplifying the scientific decision-making process. With data fast becoming a company’s most valuable asset, as workflows are automated, the data resulting from processes need to be duly managed, securely stored, and expertly analysed.
Digitalisation is the only effective way to integrate automation infrastructure with effective data management. This means physical automation systems in a lab will need to connect to digital data platforms across the enterprise. Automation without digitalisation quickly becomes unsustainable.
No matter the extent of automation employed by the organisation – at a lab or a company level – it is possible to achieve digital transformation by managing data with increasing levels of digital maturity. Listed below are the three steps to achieving digital transformation:

Figure 1: Human, physical, and digital considerations for lab automation
“Instead of managing digital disruption with apprehension, companies that plan ahead and embrace digitalisation are more likely to develop a competitive advantage in the market”
1. The Connected Lab: Connecting Data Pipelines
The foremost step towards digital transformation is to connect all the disparate data pipelines. Most organisations struggle with this fundamental step as teams work in isolation, generating siloed data that prevents collaboration. A fully connected lab is one where every functional aspect – people, equipment, consumables, workflows, and the resulting data – is seamlessly integrated.
Good quality data are defined by the FAIR attributes: findable, accessible, interoperable, and reusable. Data management through digitalisation helps achieve these characteristics across the two main modalities of data: experimental and operational.
- Connecting experimental data: Here, the raw scientific data, metadata, and processed data across all instruments are securely stored in a centralised repository. Not only are the instruments interconnected to facilitate remote monitoring, but the resulting data are also digitally accessible to perform advanced analytics or support collaboration.
Implementing a lab information management system (LIMS) is a reliable way to connect equipment and digitally manage data and workflows. Compatible with multiple vendors and automation systems, a modern LIMS tracks sample information, scientific data, and results, and stores them on cloud-based or on-site servers, in addition to providing other critical lab management functions.
With regard to personnel records, replacing manual data entries with digital documentation through electronic lab notebooks, equipped with electronic signatures and time stamps, adds user information to the scientific data, making them traceable and fully auditable. Additionally, scientific data management solutions can capture and archive instrument-related data in specific formats, with complete metadata providing context and making it available for future processing and analysis.
- Connecting operational data: Data gathered during lab operations, such as instrument calibration, system performance, servicing records, and so on, also need to be digitally stored and managed for an integrated lab ecosystem. In pharma applications, demonstrating process reliability and consistency across workflows is necessary to obtain regulatory approvals.
Moreover, real-time process monitoring via preconfigured dashboards makes it easier to identify issues or delays in the workflow and promptly troubleshoot them, vastly improving instrument uptime. Internet of things (IoT)- ready instruments, fitted with sensors synced to LIMS, enable scientists to keep track of instrument performance, and proactively schedule maintenance or take preventative measures. When combined with workflow scheduling software, scientists can exercise greater control over scientific processes and workflows. Similarly, if consumables and reagents are digitally tracked in real time by a centralised LIMS, inventory management and expense tracking also become more streamlined.
In addition to managing lab operations, audit trail features make it easier to maintain meticulous workflow records, including when an experiment was performed and by whom, enabling labs to remain compliant.
Incorporating digital informatics to capture, manage, and connect all the operational and experimental data generated in a lab significantly improves the quality of data, and, ultimately, establishes a reliable data vault. At this stage, the next step is to further boost efficiency by automating entire workflows, end to end.
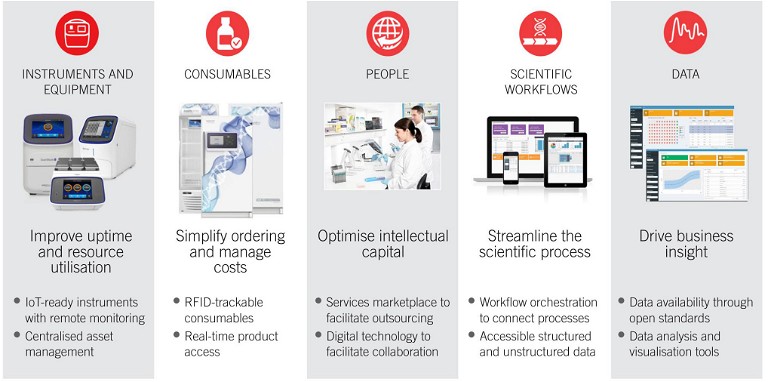
Figure 2: Digitally connecting all aspects of the lab
“Over the past decade, the surge in data production, along with advancements in computing power and training algorithms, have resulted in the rise of deep learning that, unlike ML, demonstrates more complex abstraction capabilities”
2. The Automated Lab: Improving End-to-End Workflows
Using fit-for-purpose lab workstations or robotics to automate workflows from start to finish in a digitally enabled lab catapults its productivity. Over the years, automation capabilities have gradually evolved to meet the needs of high-functioning labs. Decades ago, automation was meant to boost sample throughput to increase a lab’s overall output. As expected, the focus then was on the robotic repetition of singular tasks.
More recently, the emphasis has shifted to designing dedicated solutions for specific applications and workflows. In parallel, automated software systems have been refined to aid scheduling software and workflow management. With these developments, automation goals advanced as well, with reproducibility and accuracy taking as much precedence as speed.
Although it is possible to automate complex workflows and achieve an impressive level of lab efficiency with these dedicated automation systems, they lack one critical attribute: versatility. When experiments are modified, they require considerable reconfiguration, stretching costs and timelines. The current era of automation, therefore, focuses on being versatile to serve multiple applications and flexible to accommodate different types of lab spaces.
Configurable automation platforms typically contain a suite of modular components that can be arranged in diverse structural formats. Given this flexibility, as operations scale or locations change, the automation, too, can be easily reconfigured, thereby futureproofing the lab. Taking it one step further, it is now also possible to couple automation with virtual reality or augmented reality to develop a ‘digital twin’, where compatibility and functionality can be digitally evaluated before making an investment. Even after the automated systems are installed, labs can simulate entire workflows to optimise and fine-tune them before real samples are run, adding another layer of efficiency to the operation.
Since 2020, end-to-end workflow automation has been employed at numerous COVID-19 testing centres to expedite sample testing as the pandemic intensified. From basic machinery loading samples to sophisticated interconnected systems performing sample preparation, RNA extraction, and quantitative polymerase chain reaction, the entire workflow analyses up to 8,000 samples a day, and automatically generates reports to get test results to patients as soon as possible. At a time when testing demands were soaring, and on-site support had to be limited, this entire setup only required four supervising technicians, speeding up COVID-19 testing despite the restrictions on lab staff.
In automating workflows end to end, a capability that directly influences efficiency is the ability to walk away. Minimal human intervention decreases hands-on time for scientists. With fewer interruptions in their schedule, they can focus on high-value work that engages their full scientific potential and improves their professional experience. These factors contribute to talent retention, and the greater success of the organisation.
Results generated from automated workflows serve as immediate experimental output, but, over time, these digitally stored datasets – along with metadata – can be subjected to advanced analyses that uncover hidden trends and offer new insights. Indeed, the final step in digital transformation is to leverage one’s own data to drive future decisions.
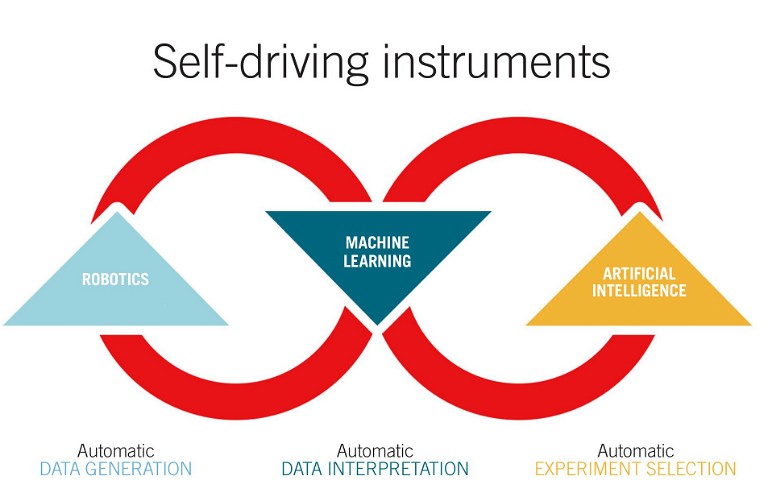
Figure 3: Closed-loop self-driving automation of the future. Adapted from a graphic from Carnegie Mellon University (2)
“When properly leveraged, deep learning has the potential to shrink timelines and minimise risks in drug development. This approach, however, is largely dependent on the foundational principles of FAIR data and connectivity”
3. The Intelligent Lab: Leveraging Data Science
After achieving the first two levels of digital transformation mentioned above, companies can dive deeper into the collected data with advanced analytics and data visualisation techniques to discover trends to guide downstream activities or future projects. As more workflows are digitally connected, higher volumes of good-quality data can be collected, gradually growing the data power of the company.
These digitally connected datasets can be eventually channelled towards artificial intelligence (AI) to serve future scientific goals. Machine learning (ML), which is essentially a subset of AI, generalises the collective information from input data, environmental signals, and feedback to produce ‘intelligence’ that can reveal meaningful insights.
Over the past decade, the surge in data production, along with advancements in computing power and training algorithms, have resulted in the rise of deep learning that, unlike ML, demonstrates more complex abstraction capabilities. Rather than generalising learned information, deep learning can apply knowledge to offer specific solutions, such as interpreting existing data or classifying new data. When properly leveraged, deep learning has the potential to shrink timelines and minimise risks in drug development. This approach, however, is largely dependent on the foundational principles of FAIR data and connectivity.
In fact, advances in data science have birthed the concept of self-driving lab instruments that can function independently. The automated systems generate data, deep learning algorithms mine and interpret these data, and, finally, AI suggests which experiments to run next, feeding it back to the automated tools in a closed self-driving loop. As these avant-garde concepts start taking shape in the near future, a scientist’s role in drug development will likely evolve as well, with intellectual contribution far outweighing experimental performance.
Automate Once, Thrive Forever
Once labs implement automation and digital connectivity, they reap the benefits of high-quality data and enhanced productivity. Companies that scale their operations in the future or open new sites will already have a firm digital footing to facilitate collaboration and data sharing. Automation, when seen through the lens of data science, also carries the potential for data-driven decision-making.
The wave of digitalisation is reaching every industry, pharma included. Instead of managing digital disruption with apprehension, companies that plan ahead and embrace digitalisation are more likely to develop a competitive advantage in the market. Whether it’s automating a single task or an entire operation, taking the necessary steps towards achieving digital transformation will ultimately accelerate drug development pipelines and manufacturing processes, bringing therapeutics to market faster.
References
1. Visit: www.accenture.com/us-en/insight-leading-new-disruptability-index
2. Visit: msas.cbd.cmu.edu

Ian Yates, Director of Enterprise Science & Innovation Partnerships at Thermo Fisher Scientific, has spent his career working at the intersection between science and technology. Beginning his career in the lab, initially as a food microbiologist, he then moved into various drug discovery roles at GlaxoWellcome (UK) and AstraZeneca (Sweden). While working in pharma labs, Ian found his professional passion – applying technology and automation to accelerate scientific discovery. This passion led Ian to move to California and join a lab robotics company, Velocity11 (later acquired by Agilent Technologies), where he led a team that delivered automation solutions globally. Joining Thermo Fisher Scientific in 2017 as a Director for the Lab Automation business, Ian is currently the Director of Enterprise Science & Innovation Partnerships. His current role creates customer partnerships that will transform the labs of the future. Ian volunteers as a career coach and mentor, and as an advocate for improving diversity and inclusion in STEM workplaces.