Analytics: Viral Vectors
Approaches to Advance Viral Vector Development and Characterisation for Gene Therapy Applications – A Journey Through Vector Optimisation
rAAV vectorology is being optimised to allow for highly specific expression and greater efficacy – how is this being achieved, and why is it so important?
Helena Meyer-Berg and Anis H Khimani at PerkinElmer
The Current State
Over the past decade, gene therapy applications have advanced across discovery, preclinical and process development workflows to become a clinical reality. Delivery of functional gene copies into tissues of interest has driven the development of viral and LNP-based non-viral vectors. Recombinant viral vectors have largely utilised either lentiviral, adenoviral or adeno-associated virus (AAV) genome backbones. Development of optimised gene expression cassettes drive controlled expression, resulting in therapeutic efficacy and better safety profiles. By now, various AAV serotypes have been widely adopted as vectors for preclinical and clinical delivery.
Based on the non-pathogenic parvovirus, recombinant adeno-associated viral (rAAV) vectors have driven numerous gene therapy programmes into clinical trials. Several successful trials have led to the approval of Luxturna® for Leber congenital amaurosis (LCA),
Zolgensma® for spinal muscular atrophy (SMA) and Hemgenix for haemophilia B, which was recently approved by the FDA.1,2 However, from other trials there have been reports of adverse effects and mortality from the high doses required for efficacy.3 At such doses, off-target expression and severe immune responses may result in toxicity and lower safety. 4
Furthermore, preclinical studies have shown correlation between dose and hepatotoxicity, neurotoxicity as well as oncogenicity potential.5,6 Therefore, engineering of rAAV therapeutic expression cassettes and capsids resulting in optimised rAAV vectors that allow for highly specific expression and offer greater efficacy at lower doses continue to be the focus of rAAV vectorology. There are ongoing efforts to develop such next-generation vectors that allow for targeted delivery.
AAV Vector Optimisation to Enhance Efficacy and Safety
Capsid engineering approaches entail rational design such as improving intracellular trafficking with 80-fold or higher efficacy or incorporating ligands in the capsid for specific targeting.7,8 Directed evolution in vivo is an alternative approach, where AAV variants are selected that have better transduction efficiency and targeted tropism after large library screening.9 A recent study combined the rational design and directed evolution approaches to generate myotropic AAV capsids, that were liver detargeted compared with rAAV9 vector.10
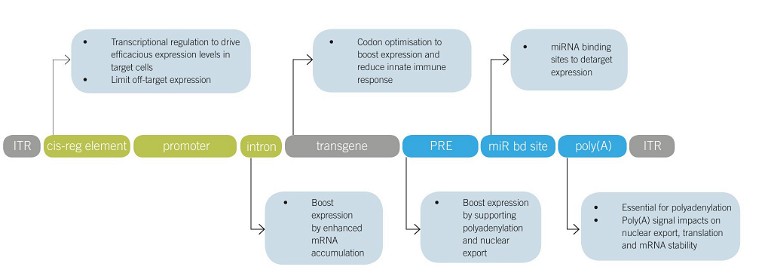
Figure 1: Therapeutic rAAV expression cassette design. Common rAAV expression cassette elements currently under preclinical or clinical investigation are shown together with their impact on safety and efficacy. Abbreviations: ITR, inverted terminal repeat; cis-reg element, cis-regulatory element; PRE, post-transcriptional regulatory element; miR bd site, miRNA binding site; polyA, polyadenylation signal
Delivery is key, but enabling efficient expression of the therapeutic gene products inside target cells is another prerequisite to engineering safe and efficacious rAAV vectors, and ensuring the potential of minimising rAAV dose. Expression cassettes are composed of multiple elements working synergistically to enable efficacious and safe expression levels (Figure 1). Although cassette design is modular to an extent, different elements impact each other. For example, the commonly employed posttranscriptional regulatory element of the Woodchuck hepatitis virus (‘WPRE’) boosted expression on multiple accounts, but this effect is not always additive in combination with promoter proximal introns.11 Therefore, experimental testing strategies need to be carefully chosen to accommodate for the multifactorial design. After the series of early rAAV gene therapy clinical trials in the late 1990s and early 2000s failed to show efficacy, the field first turned toward fixing delivery by discovering more naturally occurring AAV serotypes, as well as capsid engineering methods. With the current tremendous advancements in delivery, there is a newfound interest in expression cassette design. At the 2022 ESGCT Annual Congress, authors presented engineering of the Zolgensma expression cassette, including codon optimisation and switching from a ubiquitous promoter/enhancer to a cell type-specific promoter/enhancer element, resulting in extended lifespan and elimination of liver toxicity compared with the Zolgensma benchmark vector (OR57).12
Target cell type-specific expression can improve the safety of rAAV vectors, as it limits unintended expression and therefore toxicity. Further, unintended expression in antigen-presenting cells is linked to stronger induction of rAAV vector immunogenicity. There are several vector design strategies to limit off-target expression, eg choice of (engineered) capsid, enhancer/promoter elements and introduction of miRNA binding sites, which is a promising concept under preclinical evaluation.13 The capsid facilitates the first criterion of a successful gene therapy, which is delivery. Capsid choice can also strongly reduce off-target expression, however it often cannot be entirely circumvented due to the redundancy of rAAV entry receptors. To further fine-tune transgene expression, gene expression can be regulated by therapeutic rAAV expression cassette design. Transcriptional regulation is the most straightforward choice analogous to endogenous gene regulation, however it is often challenging to implement because of the difficulty in identifying cell type-specific enhancer/promoters and their burden on the limited rAAV packaging capacity. Enhancer elements are distal transcription factor binding sites that regulate transcription and, because they can be up to 1Mb away from the core promoter and lack common recognition sequences, they are traditionally challenging to identify. Now, with advancements in understanding gene regulatory networks and methods to study them, the gene therapy field has an entirely new opportunity to identify enhancer elements in an unbiased, high-throughput fashion.14
“ [the] engineering of ... optimised rAAV vectors that allow for highly specific expression and greater efficacy at lower doses continue[s] to be the focus of rAAV vectorology ”
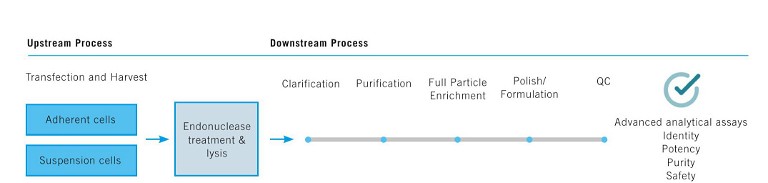
Figure 2: Upstream and downstream processing of rAAV vectors
Due to the largely modular nature of cis-regulatory elements, enhancers can be assembled with minimal promoters in synthetic promoter/enhancer complexes to enable cell type-specific expression and potentially mimic physiologic expression.
Lastly, the manufacturing process can strongly impact rAAV vector safety and efficacy. However, following ‘quality by design’ principles can ease the pressure on process development. For example, the expression cassette size as well as plasmid backbone can impact empty/partial/full/overfilled capsid ratios. The promoter choice influences if the therapeutic transgene is expressed in the production cell lines and adds to the metabolic burden on the production cells. The field has not yet constituted how an ideal rAAV capsid looks in terms of VP1:VP2:VP3 ratios, however it was already shown that engineering Rep and Cap constructs can influence VP ratios.15
Technology Trends Impacting Development, Characterisation And Scale-Up Of rAAV Vectors
While the design and engineering of rAAV vectors have been discussed above, characterisation of the rAAV is critical at every step along the upstream and downstream workflow during R&D, preclinical and manufacturing scale-up phases. A schematic of this workflow is indicated in Figure 2. Some of the major challenges during the scale-up of rAAV production are low vector titres, low full-to-empty ratios, residual host cell DNA and protein impurities that can contribute to adverse immune responses, and high yield purification of the full capsid viral particles.
Various technologies and best practices continue to be developed to meet these challenges and bottlenecks, including tools that facilitate workflow automation, data analysis and management, purification and safety metrics to meet regulatory requirements. The assessment of Critical Quality Attributes (CQAs) to ensure identity, purity, potency and stability of the vector product is important – for example, characterisation and quantification of full and empty capsids at each step. In addition, producer cell systems that can propagate the vector and provide a sustainable and scalable growth environment are desirable. A widely used cell line, HEK293, transfected with rAAV production plasmids offers a transient, scale-up system enabling levels of 10^4 up to 10^5 genome copies (GC)/cell, which drives the development of the surrounding infrastructure for workflow management, automation and regulatory compliance.
As mentioned above, AAV particle characterisation and quantification are critical to determine the ratios of full/empty with the goal to attain higher ratios. Current gold standard techniques exist, such as analytical ultracentrifugation or transmission electron microscopy (TEM), however they require large sample quantities and are laborious. Other reliable methods such as HPLC-based or mass spectrometry have been utilised, but can be costly or require a high level of expertise to implement. Recent developments enable high throughput sensitive capsid quantification with serotype specific AlphaLISA immunoassays and detailed AAV capsid protein (VP1, VP2, VP3) analysis, as well as full and empty AAV capsid characterisation and quantification from minimal sample amounts with microfluidic, capillary electrophoresis (CE) platforms.16
“ Target cell type-specific expression can improve the safety of rAAV vectors, as it limits unintended expression and therefore toxicity”
Summary
Over the past two decades, development and utilisation of viral or non-viral vectors have evolved to deliver therapeutic transgenes into cells or tissues with specificity, efficacy and better safety profiles. With rAAV vectors, research has focused on capsid engineering and optimisation for greater efficacy and safety at lower doses. Expression cassette design to enable efficacious expression levels in the targeted cells and limit off-target expression by transcriptional control is now again in the focus.
Discovery and process development workflows have leveraged technology solutions and compliance best practices from large molecule biotherapeutics environments. However, effective cellular rAAV packaging systems, streamlined scale-up and viral vector purification processes continue to develop.
For next-generation rAAV vectors, a combined approach of advanced (in process) analytical techniques, adoption of machine learning to design favourable AAV capsid variants and directed evolution, as well as promoter/enhancer elements that mimic physiologic expression hold potential for future engineering of life-changing therapies.
References

Dr Helena Meyer-Berg is a biochemist by training and completed her PhD in AAV gene therapy in the group of Prof Steve Hyde and Prof Deborah Gill at the University of Oxford. During her academic career, she developed an AAV-based gene therapy candidate for a rare, genetic paediatric disease of the lung. Since 2021, Helena has been working as an AAV viral vector platform manager at PerkinElmer’s SIRION Biotech, a company active in viral vector development and manufacturing, including high quality rAAV vectors optimised for clinical applications. As a scientific specialist, Helena drives innovation in the rAAV vector platform and is working on client projects with the goal to improve safety, efficacy and manufacturing of rAAV vectors.

Dr Anis Khimani is part of the Life Sciences segment strategy team at PerkinElmer. He has prior academic and research background in molecular biology, viral pathogenesis, viral vector development, and vaccine studies from Harvard Medical School and Dana-Farber Cancer Institute, followed by research and development in genomics and assay development at PerkinElmer. Subsequently, his focus has been in product management, business development and strategy. Dr Khimani also has industry research experience from the British multinational pharma company, The Boots Pharmaceutical Company. He has authored and co-authored numerous publications, book chapters, review articles, abstracts and has been invited to present talks at domestic and international conferences.