New Methods in Drug Development
Formulating the Design of HDASD Systems
New research indicates hot-melt extrusion produces predictable, high drug loading amorphous solid dispersions
Justin Yiwei Tian at Queen’s University Belfast, Dr Han Wu at University College London, and Robert Gurney at Linkam Scientific Instruments
The widespread adoption of combinatorial chemistry and high-throughput screening (HTS) as a means to identify promising compounds has revolutionised early-stage pipelines in drug discovery. However, because this approach does not assess dissolution and bioavailability performance of a candidate under physiological conditions, it has had the unwanted consequence that the vast majority – around 80% – of new drug candidates that emerge are classified as poorly soluble (1).
With the ideal end product of small molecule drug R&D being a once-a-day oral dose form, the pressure has fallen on formulation scientists to find ways to overcome solubility issues and advance new drugs into the clinic.
Enhancement of dissolution, solubility, and bioavailability for poorly soluble active pharmaceutical ingredients (APIs) can be achieved using a number of different approaches, including: formulation as a prodrug with good solubility that metabolises into the active compound in the body; particle size reduction to increase the surface area for dissolution (e.g., micronisation and nanomilling); salt or cocrystal formation; lipid-based selfemulsification or solid lipid nanoparticles; and the formation of an amorphous solid dispersion (ASD).
A recent review sets out the factors that affect each approach and highlights common advantages and disadvantages, depending on the specifics of the API (2). Despite the challenges that exist, developing a formulation based on an ASD is established as a popular strategy for poorly watersoluble drugs.
ASD – Challenges and Solutions
An ASD contains the API in a ‘high-energy’ state, allowing supersaturation to be achieved in the gut and increasing the absorption rate and apparent solubility. However, being in that state, the API may be unstable and can often revert to its more thermodynamically stable crystalline form over time, or in contact with biological fluids.
In addition, many existing ASD polymers only work well at relatively low drug loadings, and, as a result, many ASDs consist of 90% polymer and 10% drug by weight. This can be a significant drawback for formulation utility, in particular for lower potency drugs where it can cause higher formulation costs, inconveniently large dosage form size, or require taking multiple pills to achieve the required dose. However, drug loadings of up to 50% by weight, so-called ‘high drug-loaded ASD’ (HDASD), have been a focus of study in recent years.
It is important to note that, from a purely practical perspective, the formulation of ASDs can be achieved using well-characterised, off-the-shelf excipients, processes, and screening methods in order to differentiate and select formulations according to their in vitro, and likely in vivo, behaviour. For example, the processes typically used to produce ASDs, such as spray drying and melt extrusion, are well established and scalable to commercial quantities.
As a consequence, these three elements are the focus of attention for those wanting to utilise this approach – overcoming the innate stability issues, achieving high drug loading, and simplifying processing and manufacture.
Exploring HDASD by Hot-Melt Extrusion
Recent work by a team of researchers at Queen’s University Belfast, Northern Ireland and University College London, UK has reported on a newly developed strategy for the manufacture of an HDASD using a hot-melt extrusion (HME)-based platform (3).
In general, spray drying and cryo-milling are recognised as the preferred techniques for producing these HDASD systems, though, as they are inherently unstable, limitations remain (4). As an alternative, hot-melt extrusion platforms are coming under the spotlight. The work led by Yiwei Tian and Gavin Andrews, investigated the properties of one particular category of ASD, and explored the formation of HDASD via a HME platform.
Previously, it has been shown that strong intermolecular interaction between drug and polymer can provide the main mechanism for amorphisation during high-temperature processing. A significant increase in the drug loading and amorphous stability is commonly reported for these systems.
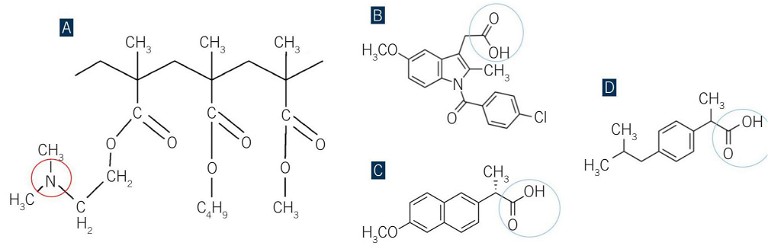
Figure 1: Chemical structure of polymer a) Eudragit EPO, b) and drug indomethacin, c) naproxen, d) ibuprofen, reported potential strong drug-polymer inter-molecular interactions are highlighted in circles
Building on this knowledge, the thermodynamic characteristics of formulation design integrated with HME platform were investigated. The weak acidic drugs (indomethacin, ibuprofen, and naproxen) with amorphous polymeric carrier Eudragit®E(EPO) were chosen as suitable combinations for study (see Figure 1). Thermodynamic phase diagrams for three HDASD systems were constructed and utilised to guide the design of the HME process and the processability and storage stability of HDASDs were further investigated using a range of conventional and advanced analytical techniques.
Methodology: temperature-dependent small angle/wide angle X-ray scattering (SAXS/WAXS)
Yiwei Tian explains the methodology used: “The solid-state properties of ball-milled samples and the dissolution/melting behaviours of crystalline drug in the polymeric matrix, as a function of temperature, were analysed using SAXS/WAXS. The temperature control of the sample was realised by a heating and cooling stage, with controller and liquid nitrogen cooling system, installed inside the vacuum chamber. The SAXS/WAXS system was calibrated without the need of a standard. The calibration was validated before each measurement using lanthanum hexaboride.”
In a typical temperature-dependent SAXS/WAXS experiment, samples of around 20mg were packed in an envelope of aluminium foil, secured on the centre of the heating plate, and subjected to a heat-cool-heat cycle similar to a differential scanning calorimetry (DSC) experiment. The sample was heated from 25°C to 160°C at a rate of 5°C/min, then fast cooled to -40°C at a rate of 40°C/min. A second heating procedure was conducted from -40°C to 160°C at a rate of 5°C/min.
Yiwei Tian continues: “The WAXS was collected every 60 seconds at exposure time of 30 seconds during the heat-coolheat cycle. SAXS was also collected on selected samples with 600 seconds of exposure time at key temperature points.”
Further analysis of the samples included HME, thermal analysis, attenuated total reflectance Fourier-transform infrared (ATR-FTIR) spectroscopy, atomic force microscopy (AFM), and microRaman spectroscopy.
Experimental Partnership
Once the protocol for the experiments was set, Yiwei Tian partnered with Han Wu, Research Lab Manager at the Centre for Nature-Inspired Engineering (CNIE) research facility at University College London, UK to make the SAXS/WAXS measurements. The CNIE was established in 2014 to support multi-disciplinary research across the University, and for industrial collaborators and other academic users.
Yiwei Tian comments: “Having met Han Wu at a crystallisation conference and heard about her facility, I knew that the team at CNIE had the skills – and the equipment – Ineeded to realise my work. The SAXS/WAXS setup there, together with a Linkam stage for precise temperature control and rapid heating and cooling, is ideal for time-resolved studies of the phase behaviour of pharmaceutical compounds.”
Results
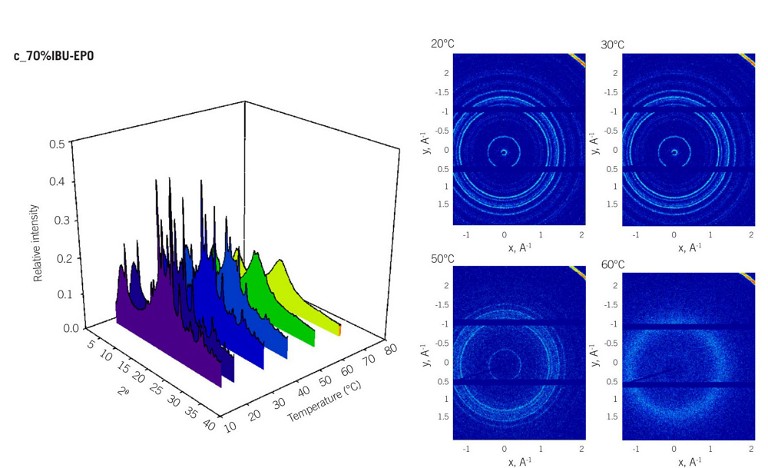
The study was able to directly observe the temperature dependences of the X-ray scattering pattern for the crystalline drugs (indapamide drug [IND], ibuprofen ketoprofen naproxen [NPX], and ibuprofen [IBU]) in the presence of a polymeric carrier. As seen in Figure 2, page 12, the dissolution of the crystalline drug into polymeric matrix results in the decrease of scattering intensity in the WAXS trace and resultant spectrum, and eventually, complete loss of the welldefined scattering pattern.
Examples of 3D Bragg’s peak for the physical mixtures (three drugs at 0.7 w/w) as a function of temperature are also shown for each mixture, with WAXS scattering traces provided at four key temperature points. Due to the strong intermolecular interactions between drugs and the polymeric carrier erythropoietin (EPO), a gradual decrease in the intensity of Bragg’s peaks associated with the crystalline component was observed with increased temperatures.
Finally, Figure 2 also shows that in IND-EPO physical mixtures, the temperature for complete dissolution of 0.7 w/w IND was recorded at 125 to 130°C, approximately 30°C lower than the melting temperature of pure IND (DSC, 160°C). For NPX-EPO and IBU-EPO systems containing 0.7 w/w drug loading, the solubilisation temperatures were recorded at 120-125°C and 50-55°C respectively.
Yiwei Tian comments on the significance of the data: “This information is particularly useful for the design of HME process, where a fully amorphous solid dispersion can be generated at conditions that are significantly lower than the melting of the crystalline drug, and at much higher drug loadings. The unwanted thermodegradation associated with melting of the drug may be completely avoided since the amorphisation of crystalline drug is driven by the dissolution, rather than melting.”
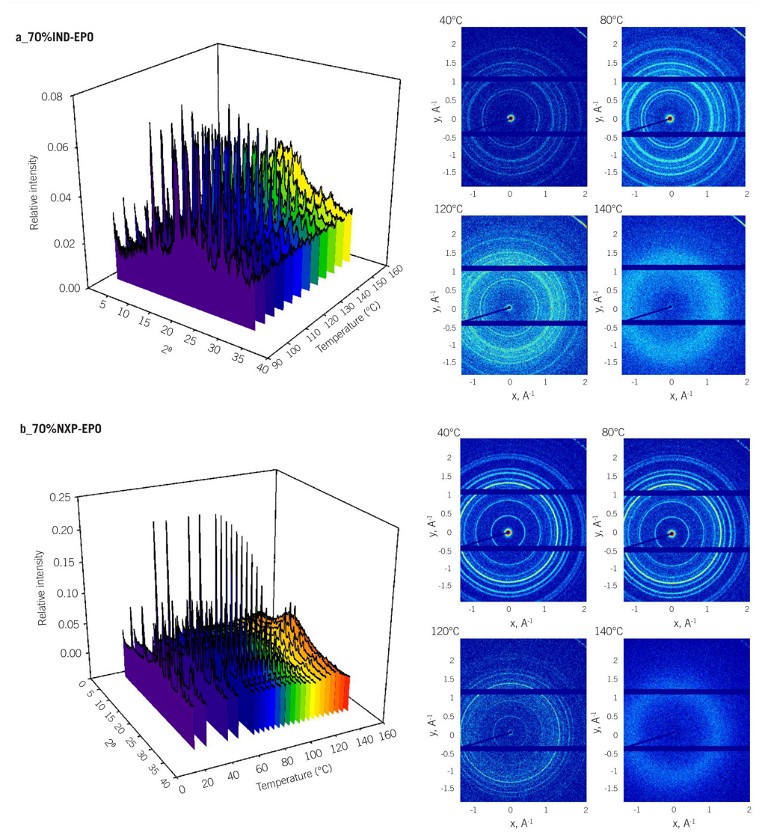
Figure 2s: a) IND-EXP, b) NPX-EPO, c) and IBU-EPO, at drug weight fraction of 0.7 w/w
Conclusions
SAXS/WAXS analysis in conjunction with the other measurements and theoretical modelling that are reported in the recent publication, strongly indicate the possibility of forming stable amorphous systems at high drug loadings and low temperatures.
Additional characterisations on the extrudates confirmed that the amorphisation of NPX, IBU, and IND at these predefined conditions can be achieved by one-step continuous HME process, and the establishment of a predictive thermodynamic model allows the interpretation and evaluation of the HME process in a well-defined space, ensuring the quality of amorphous solid dispersions.
Further work is planned by the researchers, to investigate the kinetic impacts on the HME process based on the predefined thermodynamics of the HDASD systems.
References

Dr Justin Yiwei Tian BEng, MSc, is a Lecturer (Assistant Professor) in Pharmaceutical Engineering Group, Queen’s University Belfast, Northern Ireland, and was a Royal Academy of Engineering Enterprise Fellow, UK (2019/20). He has completed his Bachelor’s degree in the area of Macromolecular Materials and Engineering from Beijing Institute of Technology, China, an MSc in Process Engineering, and a PhD in Drug Delivery.

Dr Han Wu is a Research Lab Manager at University College London (UCL), UK, Chemical Engineering, managing a range of research facilities including SAXS/WAXS. Han obtained her PhD at the University of Sheffield before moving to UCL and worked as a Post-Doctoral Research Associate on crystallisation of pharmaceutical materials. Han is a committee member of the Particle Characterisation Interest Group at the Royal Society of Chemistry and provides training and conferences to the university researchers. She is an active member of ISO/TC24/SC4 (WG10-SAXS) for the development of particle characterisation standards using SAXS method.

Dr Robert Gurney is Marketing and Applications Specialist, at Linkam Scientific Instruments. Robert joined Linkam’s Sales and Marketing team in 2019 and has expertise in a range of scientific techniques for temperature, environmental, and mechanical sample characterisation. He is responsible for Linkam’s scientific articles and marketing materials. Robert obtained his PhD in Soft Materials from the University of Surrey, UK, and has worked at The City of Paris Industrial Physics and Chemistry Higher Educational Institution, France, and Wuhan University of Technology, China, on projects including thin film analysis, chemical synthesis, and solar cell characterisation.