Discovery & Development: Inhalation
An Alternative Regulatory Pathway for Generic Orally Inhaled Drug Products
The in vitro-in silico ‘alternative’ bioequivalence pathway for generic inhaled drug products, and how to factor in patient variability to successfully replace your comparative clinical end-point study
Jan de Backer at FLUIDDA, and William Ganley and Gemma Budd at Nanopharm
Challenges & Context
The current regulatory pathway for generic orally inhaled drug products (OIDPs) is challenging, due to the cost and time associated with meeting the clinical end-points, and the low success rate (1). Yet, the FDA does not have an established alternative to demonstrate bioequivalence.
However, the FDA has been actively funding the development of technologies to enable in vitro-in silico approaches and have even updated some Product Specific Guidance (PSG), introducing the “alternative approach to the comparative clinical end-point BE study (CCEP BE)” (2, 3). While this demonstrates the regulator’s openness to potential alternative approaches, the PSG are not explicit with protocol expectations. Indeed, the FDA “strongly encourage” sponsors to discuss this via the pre-ANDA meeting pathway, suggesting that the requirements are still not completely defined and the onus is on the pharma companies to propose the approach.
The main challenge associated with evaluating these factors in CCEP BE is that patient-to-patient variability has such a significant impact on outcomes. This typically means that cohorts of 1-2,000 patients, over a number of weeks, are needed to build enough power for the statistical analysis, which quickly becomes very costly (4). Furthermore, it is difficult to replicate and discriminate patient variability in a way that determines whether similarities or differences are the result of the patients’ (in)ability to use the product as intended, the difference in the patients’ diseased lung physiology, or whether it is a fundamental difference between products themselves. The latter is primarily what the regulatory bodies must objectively assess and is difficult to achieve in a non-clinical setting.
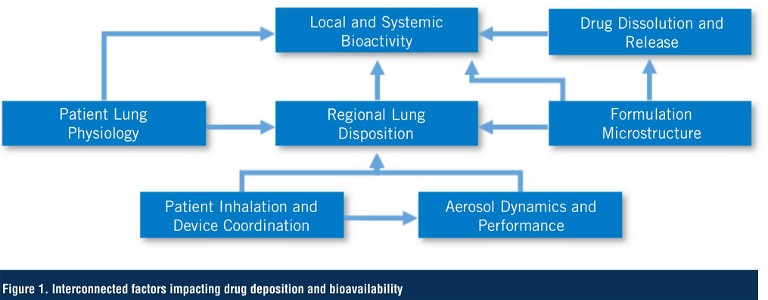
For example, the patients’ coordination between actuating the device and inhaling the product – and their inhalation profile – impacts the amount of drug delivered from the device, its regional exposure in the lung, and ultimately its availability at the site of action (5). This, in conjunction with inherent variability between product batches, potential differences in the physicochemical properties of the APIs and excipients, interactions between them, and manufacturing process, creates a matrix of factors that can heavily influence performance (Figure 1). Most in vitro and in silico platforms use idealised conditions – essentially representative of the ‘median’ healthy population with some, often arbitrary, variations – and these are helpful screening tools which can be useful in directing product optimisation. However, to say this provides a prediction of clinical performance is comparable to suggesting that you could dose a single patient in the clinic that demonstrates the ‘median’ attributes of a population and expect the clinical result to be the same as if you dosed 2,000 patients.
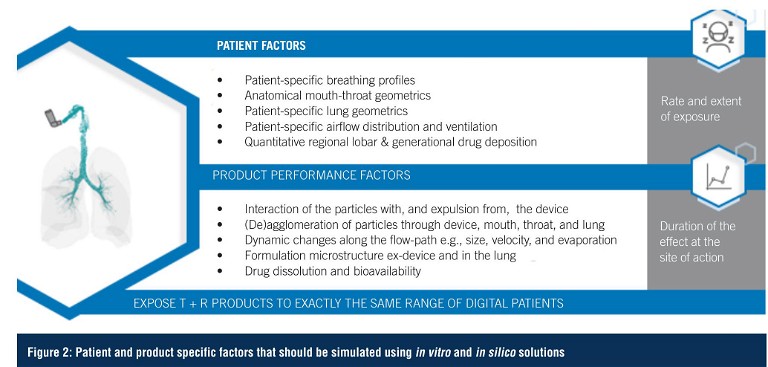
Technology Solutions
To mitigate this, any approach needs to follow the drug from the device to the lungs, reproducing the dynamic environment along its path. Importantly, since all drug products are different, there cannot be a one-size-fits-all approach, hence an understanding of drug mechanisms and patient physiology are just as important as controlling the chemistry, manufacturing and controls (CMC). However, provided both patient and product factors are considered consistently throughout a study (Figure 2), a clinically-relevant result can be generated.
The ability to capture inhalation profiles from the patient population using the target devices, and the subsequent ability to recreate these during in vitro testing, such as aerodynamic particle size distribution (APSD), is essential. In combination with anatomical mouth-throat (MT) models, this allows for the prediction of the fine particle mass and fraction with a much stronger in vitro-in vivo correlation (IVIVC) than traditional USP throats (6, 11). The total lung dose can also be collected using an aerosol dose collection system, preserving the product in its aerosolised state for microstructural (Q3) assessment using simultaneous morphology-based image analysis and chemical identification tools such as Raman spectroscopy, to quantitatively and qualitatively assess all components in the formulation along the airflow path (7, 2). In conjunction with an in vitro assessment of the dissolution rate of the drugs, a credible assessment of the rate and extent of release of the drugs at the site of action can be obtained (8).
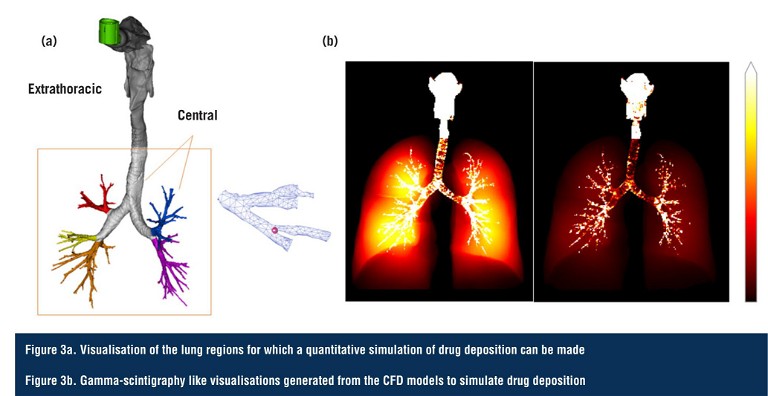
These breathing profiles and particle properties are incorporated into computational fluid dynamics (CFD) meshes, which are coupled to quantitative high-resolution computed tomography (HRCT) scans of real patient lungs measured at inspiration and expiration. The in vitro data feed the CFD models to enable the quantitative prediction of regional drug exposure in the different lobes and generations of the disease-state lungs (Figure 3).
Such patient-specific CFD models have already been validated against scintigraphy data for a number of drug products, and can be further validated by confirming that their predictions align with the in vitro measurements of ex-throat dose and lung dose (using the MT models), which have themselves been clinically validated (9, 10). This approach could conceivably be the basis of a ‘digital twin’ for respiratory diseases; a concept that is increasingly gaining traction in the medical community.
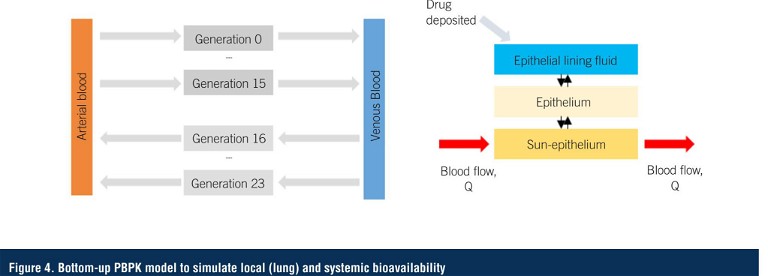
The quantitative regional lung deposition data generated by CFD, alongside inputs from the previous in vitro studies such as dissolution, are fed into a physiologically based pharmacokinetic (PBPK) model for each disease state (Figure 4), to simulate local lung kinetics and bioavailability, and subsequent absorption into the bloodstream (11, 12, 13).
Whilst this approach provides more clinically relevant data, patient variability still needs to be accounted for. In a clinical study, you rely on random recruitment to capture variability across a patient population, which results in large numbers. Through design of experiments, this in vitro-in silico approach can cover the complete range of patient-to-patient variation in a fraction of the cohort size, and produce a more robust result. Any difference can be clearly attributed to a certain factor and the relative influence and interconnection between multiple differences on the outcome can be modelled. This not only allows for a credible prediction of bioequivalence, but can also help to determine which critical quality attributes (CQA) are most clinically significant for each drug product within the context of efficacy.
Risk-Based Framework
As the influence of the patient needs to be represented and simulated throughout all tests performed, each data output needs to feed into a subsequent measurement (whether in vitro or in silico) – whilst being independently validated – in order to build the credibility of the platform. Since the regulators still require a pharmacokinetic (PK) study on healthy volunteers (or, in some cases, patients) – and this is predicted by the PBPK model – the alignment of the clinical PK data with the in silico predictions offers further validation of the credibility of the models. This reduces the risk profile associated with applying the same models to patient-specific studies. However, greater reliance on in silico predictions can increase risk because less clinical evidence is being generated. This is a particular challenge when considering a drug with its site of action in the lungs, predicting phenomena that cannot directly be measured.
The risks of adopting an in vitro-in silico approach to demonstrating bioequivalence can be managed through the FDA-recommended V&V40 framework (2). On this basis, it becomes obvious that greater integration between the different factors being measured or simulated results in lower risk of the resulting predictions being unreliable.
Using this framework, the question of interest (QOI) can be defined in the context of the alternative bioequivalence assessment: ‘Is the rate and extent of drug exposure and availability of the test and reference product within acceptable limits of bioequivalence, and thus sufficient to justify a clinical end-point study biowaiver?’
“ The main challenge associated with evaluating these factors in CCEP BE is that patient-to-patient variability has such a significant impact on outcomes ”
The context of use (COU) is a more detailed statement defining the scope and specific role of the in vitro-in silico modelling in addressing the QOI. As previously mentioned, the strategy is to use CFD to simulate the regional lung deposition and subsequent bioavailability of the drug products in patient-specific airways from the patient demographic (generated using Functional Respiratory Imaging), coupled with physiologically based pharmacokinetic modelling (PBPK) to predict the local rate and extent of drug activity in the lung tissues. The models would be used in conjunction with the realistic aerodynamic particle size distribution (rAPSD) measurements with anatomical mouth-throat (MT) models and patient-specific breathing profiles, aerosol agglomeration and de-agglomeration profiles, dissolution and permeation rate of the drug substances, and microstructure and morphology imaging comparisons.
The objective is to maintain the principles of a ‘weight of evidence’ strategy utilised by the FDA, where no claims of bioequivalence will be made based on isolated data, resulting in the ‘model influence’ being ‘low’. Optimally, the in silico predictions will not only complement the in vitro test results, but each set of data will be interconnected such that there are very few independent datasets that make unique claims. This could arguably define the ‘decision consequence’ risk as ‘medium’, particularly since the safety profiles of the compounds are already well-established. By contrast, in a scenario where each dataset is unique and the sponsors simply follow a tick-box exercise similar to the existing in vitro bioequivalence (IVBE) studies that are performed, one cannot derisk or validate the other, meaning that the model influence and decision consequence would be potentially ‘medium-high’.
Conclusion
Taking this approach not only provides regulators with the confidence they need, but also provides a robust tool to help with the development and optimisation of a product from the outset, since the studies can help to set a target product profile with significantly more substantial datasets to build from. The challenge of being an innovator is that there may be limited fundamental understanding of your product and what makes your drug product work. This is not to undermine the extent of complex research that goes into developing a novel product, but once a product is shown to work, the emphasis shifts towards addressing process engineering challenges rather than continuing to explore the science. For a generic company that doesn’t have visibility of a product’s development history, they arguably need to develop an even deeper understanding of the critical performance attributes of the product, and, importantly, how they contribute to the clinical result. One could say that generic companies are now in a position to understand the product science better than the innovator by deploying the tools outlined in this approach.
The challenge has always been how to holistically capture the impact of the patient, and how to simulate what you can’t measure. The combined approaches discussed above significantly close that gap, and can provide regulators and sponsors with the confidence that a product is fit for purpose, enabling an accelerated and, arguably, more objective regulatory pathway. The outcome offers an opportunity to go beyond the current expectations of the industry and regulatory bodies, and proposes an incontrovertible approach that may arguably be proven to be less risky than performing the existing clinical end-point studies and, in time, become the recommended approach rather than only the alternative.
“ Taking this approach not only provides regulators with the confidence they need, but also provides a robust tool to help with the development and optimisation of a product from the outset ”
References
- Visit: www.fiercepharma.com/pharma/fda-sends-hikma-vectura-back-to-clinic-advair-copy-now-due-2020
- Visit: www.fda.gov/media/156481/download#page=39
- Visit: www.accessdata.fda.gov/drugsatfda_docs/psg/Beclomethasone%20dipropionate%20Inhalation%20Aerosol%20Metered%20NDA%20207921%20PSG%20Page%20RC%20May%202019.pdf
- Visit: www.clinicaltrials.gov/ct2/show/NCT02649478
- Van Holsbeke, C et al, Use of functional respiratory imaging to characterise the effect of inhalation profile and particle size on lung deposition of inhaled corticosteroid/long-acting beta-2 agonists delivered by a pressurised meter dose inhaler, Ther Adv Respir Dis, 2018
- Kaviratna, A et al, Evaluation of Bio-relevant Mouth-Throat Models for Characterisation of Metered Dose Inhalers, AAPS PharmSciTech 20(130), 2017
- Price, R et al, Development of an Aerosol Dose Collection Apparatus for In Vitro Dissolution Measurements of Orally Inhaled Drug Products, The AAPS Journal 22(47), 2020
- Kumar, A et al, A Biocompatible Synthetic Lung Fluid Based on Human Respiratory Tract Lining Fluid Composition. Pharm Res 34: pp2454-2465, 2017
- Vinchurkar, S et al, A case series on lung deposition analysis of inhaled medication using functional imaging based computational fluid dynamics in asthmatic patients: effect of upper airway morphology and comparison with in vivo data, Inhalation Toxicology; International Forum for Respiratory Research 24(12), 2012
- Wei, X et al, In Vitro Tests for Aerosol Deposition. VI: Realistic Testing with Different Mouth–Throat Models and In Vitro—In Vivo Correlations for a Dry Powder Inhaler, Metered Dose Inhaler, and Soft Mist Inhaler, Journal Of Aerosol Medicine An, Journal Of Aerosol Medicine And Pulmonary Drug Delivery 31(6): pp358-371, 2018,
- Ganley, W et al, Investigating the Regional Deposition and Simulated Inhaled Pharmacokinetic Profiles of Generic Dry Powder Inhalers, Drug Delivery to the Lungs, 2019
- Hochhaus, G et al, Can Pharmacokinetic Studies Assess the Pulmonary Fate of Dry Powder Inhaler Formulations of Fluticasone Propionate? AAPS J 23(48), 2021
- Rossi I et al, The Role of In Silico Regional Deposition Modelling and Pharmacokinetic Profiling in the Development of a Generic Tiotropium Dry Powder Inhaler, Respiratory Drug Delivery, 2020

Jan De Backer graduated from Delft University of Technology, The Netherlands, as an aerospace engineer. He attained an MSc degree in Aerodynamics and specialised in Applied Biomedical Computational Fluid Dynamics leading to a PhD from the University of Antwerp, Belgium. He is an alumnus of the MBA programmes at London Business School, UK, and Columbia Business School, US. Dr De Backer has received several awards for his innovative research in the field of airway modelling in respiratory and sleep medicine. His work has been published in international journals. Dr De Backer founded FLUIDDA in 2005 and he has held the position of Chief Executive Officer since 2007.

William Ganley is Head of Computational Pharmaceutics at Nanopharm Ltd, an Aptar Pharma Company, where he is responsible for developing in silico methodologies and data analytics services to support OINDP companies through clinical development to regulatory approval. Holding an MChem from the University of Southampton and a PhD from the University of Bristol, both UK, Dr Ganley has presented work on physical chemistry and pharmaceutics at multiple international conferences and published several peer-reviewed articles. He was awarded the Faculty of Science Prize for the Physical Sciences and the Best Doctoral Research Thesis Prize for the Faculty of Science for his PhD.

Gemma Budd is Business Development Director for Nanopharm Ltd, an Aptar Company, where she is primarily responsible for developing business and collaborative opportunities for Aptar Pharma’s services, solutions and technologies in the orally inhaled and nasal drug product (OINDP) sector. With a university degree in Biomedical Sciences, Gemma has over 10 years of experience in the pharmaceutical industry in both research and commercial positions, from materials science and analytical services, to formulation technology and drug delivery devices, primarily for oral and inhaled dosage forms.