Preclinical Imaging
Advancements in preclinical imaging: a look into the future of drug development
IPT talks with Jacob Tesdorpf at Revvity about the recent developments in preclinical imaging, from AI-enchanced analysis to refined disease models
Jacob Tesdorpf, Revvity
IPT: Over the last five years, what have been the most groundbreaking developments in preclinical imaging technologies?
Jacob Tesdorpf (JT): Animal experimentation always needs to balance the need for scientific advancement with ethics and address the translatability of results between species. The research community has therefore adopted the 3R principles striving to replace, reduce and refine those experiments. Pre-clinical imaging plays a crucial role in these efforts as it allows for less animals per experiment and offers non-invasive ways to measure outcomes.
Today researchers can access preclinical versions of all clinical imaging modalities (eg, ultrasound, X-ray, CT, MRI, PET) and benefit from dedicated preclinical modalities such as optical imaging that offer functional insights not readily available in a clinical setting.
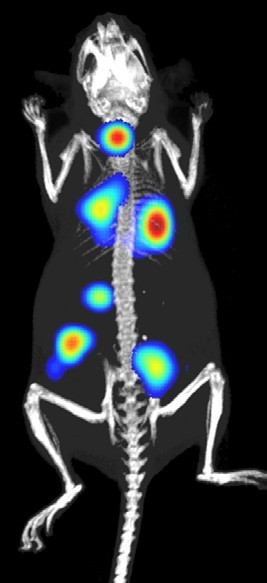
The depth of information researchers can extract from every experiment has fundamentally changed over the last five years to provide greater clarity for decision-making in drug development. This has been driven by improvements in instrumentation, software and probes. One example of this is combining anatomical and molecular images into a single data set, which enables more accurate assessment of disease progression in response to treatment.
Traditionally, assessing the impact of cancer treatment on tumour growth was measured manually with callipers. This approach, in many cases, has been replaced by image-based qualification of luminescent cancer cell mass, or ultrasonic evaluation of tumour volume. And more recently, researchers are adding fluorescent markers of functional response and co-registering images with a CT scan to provide anatomical context to the quantification. These imaging approaches have allowed researchers to observe the progression of tumours located deeper in the body (eg, orthotopic tumours) which are difficult to reach by callipers. Another advanced research protocol recently deployed allows for tumour’s borders to be accurately traced from whole-body high-resolution 3D ultrasound images, regardless of the tumour’s location or possible abnormal structure.
This had led to more accurate volumetric sizing of tumours over time and allows newer models such as patient-derived xenograft tumours to be tracked without needing to label the cells.
IPT: What are some difficulties you have had to deal with?
JT: When thinking about the path from targets to cures there are big questions: how to address unmet clinical needs (in this case, novel therapeutic modalities may offer hope), how to better predict clinical efficacy in preclinical experiments (which may require better disease models and more comprehensive data) and how to accelerate time to market (which can be addressed by streamlined workflows and automation)? One of the challenges that remain in preclinical imaging is standardisation as a recent survey found.1 This is in part a task for scientists to implement respective quality guidelines and processes, but suppliers can help through design. A good example might be the first preclinical ultrasound system to automate the current manual procedure thereby reducing operator variability.
IPT: How has the use of AI helped to further develop and improve preclinical imaging?
JT: AI and machine learning methods are aiding image segmentation and classification of preclinical images that contain a huge amount of information, which characterises response to treatment. This in turn is reducing the amount of time researchers are spending on manual, often qualitative identification of features resulting in faster, unbiased drug development. Recently AI is being applied to help not just analyse results but also to use subtle features to predict outcomes.
This leads to faster drug development and unbiased qualification. The ability to apply AI to image data analysis for AI-assisted segmentation and regions of interest (ROI) assignment can greatly advance and accelerate the field of preclinical imaging. If researchers can compress the time required for analysis, they can now use this additional time to either collect more data, run more experiments, test additional formulations of a therapeutic compound, etc. By reducing the widely held bottleneck of manual image analysis time from thousands of preclinical research labs across the world, we hope to enable significantly more productive science to be done per day.
AI development requires thousands of examples of training data to build the algorithms to detect disease within preclinical image data (similar to how humans can be trained to read images, so do computers need lots of repetition to get sufficient accuracy and sensitivity). We are partnering with customers to build these AI algorithms by contributing portions of their extensive libraries of previous studies they have run and analysed with traditional manual approaches. In turn, customers are empowering our AI research capabilities without requiring anyone to do any additional work. In other words, we are building significant future innovations hand in hand with our customers on a foundation of real-world image libraries from recently performed research studies from their labs. In return, these customers gain access to cutting-edge tools that have received bespoke training on their specific disease areas of focus and image protocols.
IPT: Can you tell us more about how recent advances in preclinical imaging technologies have benefited patients and diagnostics?
JT: Recent preclinical technology enhancements are lowering the limits of detection and increasing the depth at which information can be obtained from within the subject. This is allowing researchers to work with tissues cultured directly from patients and position them in orthotopic locations resulting in model systems which are better at predicting clinical responses.
Better clinical predictions mean better biomarkers, more targeted treatments with fewer side effects and ultimately better patient outcomes. For example, recent studies have shown orthotopic-implantation of patient-derived cancer cells in mice recapitulates clinical response in patients and could therefore be used to guide therapeutic decision-making.
Another field where non-invasive imaging tools are likely to make an impact is regenerative medicine.2 While nascent, this is a growing area of scientific interest and industry investment given the outsized demand for transplantable organs.
For example, in engineered bio-implantable tissues, imaging technologies could be used to assess sample viability and safety prior to implant. Traditional histological methods of assessing sample viability are destructive by nature, and thus not suitable for quality control (QC) of these highly sensitive implantable organs and tissues.
Even before bio-implantable tissue technologies are ready for human application, imaging tools can help drive innovation in this space by allowing researchers to optimise their tissue engineering protocols and evaluate the results with a faster feedback loop (seeing the results in real time vs waiting weeks for histologic workup).
Imaging could also be leveraged in precision medicine efforts or developing new cell therapies.3, 4 Researchers in these fields can evaluate tumour molecular and functional metrics before, during and after therapy in a longitudinal manner. This can help to accelerate discoveries such as why certain tumours respond to novel cell and gene therapies and others do not, tuning the optimal dosing and delivery timelines and evaluating which biological processes underpin drug resistance (by timing study endpoints to inflection points in tumour growth, when the dynamics of the microenvironment rapidly shift).
IPT: How do you see the field of preclinical imaging developing in the next five years?
JT: The proportion of genomic medicines in the drug development pipeline continues to grow as well as reliance on technologies such as preclinical imaging to test and validate functional response to molecular alterations. In order to meet this increased demand, I expect there will be increased reliance on automation to both provide rapid, consistent capture of high-quality functional and anatomical images and also to automate the analysis pipeline, making use of AI and machine learning to segment and analyse and predict response.
As a result of improved automation across the entire life sciences market, preclinical imaging will see a dramatic shift toward faster and more user-agnostic workflows that increasingly accelerate and standardise acquisitions and analysis. This will allow different labs to more easily compare data sets and collaborate more effectively. Additionally, improved automation will reduce the barrier to entry for new users by eliminating bottlenecks that exist within some imaging modalities, further driving discoveries by allowing wider impact of these powerful imaging tools. This combination of streamlining and better clinical predictivity means the right, novel or even curative therapies are progressed through development pipelines faster to overcome the world’s greatest health challenges.
References
- Mol Imaging Biol. 2023 Jun;25(3):560-568.
- Visit: doi.org/10.1038/s41536-017-0029-9
- Visit: doi.org/10.3390/tomography9030076
- Visit: doi.org/10.1073/pnas.2221535120

As Life Sciences Market Segment leader Dr Jacob G Tesdorpf is leading Revvity’s segment strategy team for the life sciences markets. Since joining Revvity he led product management and application teams for plate readers, high content screening and preclinical imaging.