Tech in Cell Line Development
Advancing cell line development: how next-gen innovation meets industry needs
Cell line development is integral to research and biotherapeutic production, and so ensuring this process is as robust as possible through next-generation technology is in the industry’s best interests
Maryam Ahmadi at Sphere Fluidics
The global biopharmaceutical market is projected to grow from $617bn in 2024 to $1,184bn by 2032, demonstrating a compound annual growth rate (CAGR) of 8.5%.However, to meet growing demand, there is increasing pressure on the industry to streamline biotherapeutic discovery, and to maximise the efficiency, quality and safety during biotherapeutic production, including mono-specific and bi-specific monoclonal antibodies, fusion proteins and vaccines. 1
Cell lines are fundamental to producing the majority of today’s biotherapeutics, enabling researchers to tailor cultures to align with the desired product properties. Their use has also revolutionised scientific research by offering considerable advantages over primary cells, including cost, ease-of-use, providing an unlimited material supply, and monoclonality assurance to ensure reliable and consistent outcomes. As a result, robust cell line development (CLD) is crucial to facilitate research and biotherapeutic production.
To improve the success rates and efficiency of biopharmaceutical production, early developability assessments are essential. These assessments focus on optimising the selection of high-yield and stable cell lines capable of producing the target protein efficiently. An effective selection process not only minimises time, effort and costs, but also enables the identification of clones that are unlikely to succeed during scale-up, thereby allowing for early intervention in the development process.
Furthermore, in cell engineering workflows, there is a need to rapidly screen large heterogenous cell populations to enable researchers to enhance metabolic pathways, produce key enzymes and metabolites, or create new molecules.
Quality assurance in CLD is essential, particularly if their intended use is for biotherapeutic production. There are rigorous regulatory requirements set by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) to ensure the safety and efficacy of therapeutics, and assess the quality and consistency of cell lines. These encourage the industry to explore and embrace novel approaches and technologies that facilitate the highest standards of performance.
Droplet microfluidics for single-cell analysis
A well-established, scalable and cost-effective method for rapid analysis, characterisation and selection of single cells is the use of droplet-based systems. This technique involves encapsulating cells in droplets of suitable cell culture media suspended in an oil carrier fluid. By controlling the initial cell density, droplets occupied by single cells can be generated, enabling single-cell analysis (Figure 1). When combined with a fluorescence detection system, this approach can be applied in CLD to automate the screening of large, heterogeneous cell populations, allowing for the identification and selection of individual cells with specific phenotypes or features of interest while preserving their viability.
Technological innovation in this field, in particular, is rapid.
Challenges in CLD and how technology is innovating optimisation
Although cutting-edge advances in cell line engineering and automation technologies have significantly reduced CLD timelines, generating high-producing, stable cell lines can be challenging due to increasing complexity of drug design.
It is critical to take into consideration a deeper understanding of where any new challenges or potential barriers may arise, and how to overcome these to deliver both speed and precision in CLD.
Host cell line selection bias
During CLD, selection bias in the host cell line can occur if clones are enriched based on stability or antibody secretion level at the time of measurement. This approach can be problematic later in the development process as these factors do not guarantee future productivity, production or growth stability. To mitigate this, it is beneficial to select cells based on their potential for future performance. This can be accomplished by evaluating a variety of highand medium-producing clones through their performance during expansion. These validation steps help to ensure the most suitable clones with advantageous attributes for biopharmaceutical production are selected early on.
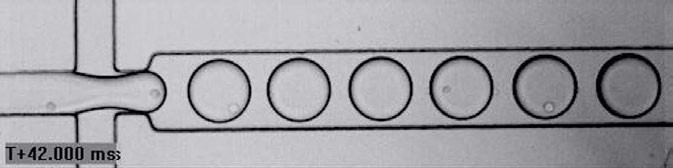
Figure 1: Droplet generation process to encapsulate a single cell per droplet
When using droplet-based systems for CLD, the droplets serve as precisely defined and controlled micro-reaction chambers in which the individual cells can be assayed for productivity. This also enables the cells to be easily isolated and expanded to assess growth stability and ensure they can be scaled for production.
Unpredictable gene expression
Gene expression during CLD can be unpredictable despite efforts to optimise the host cell line, either through directed evolution or using chemically defined, animal-free media.
However, gene expression can be regulated and improved using various techniques. For example, selection of cells with the desired expression levels can be more difficult if the expression of a gene of interest is solely reliant on random integration methods. One approach to tackle this issue is through the use of site-specific integration, which has gained significant traction with the advances in genome engineering, including the development of nucleases such as clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9), transcription activator-like effector nucleases (TALENS) and zinc-finger nucleases (ZFNs).
Site-specific integration
Researchers are now able to target specific regions to control the site of integration; improving recombinant gene expression, reducing clonal diversity and achieving more dependable expression. Targeting to specific regions such as Hipp11 and Rosa26 have been investigated as ‘safe harbour’ locations for gene insertion in mammalian cell lines and have been shown to increase integration and stable gene expression.2,3 Despite their advantages, adopting technologies like CRISPR has potential drawbacks as they may generate off-target effects and create mutations in the host genome.
However, advances in CRISPR technology are increasing its functional capabilities, reducing off-target effects and minimising mutation events.
Expression vector design
Gene amplification can significantly enhance a cell line’s productivity, making expression vector design essential for developing high-producing cell lines. These vectors typically include genes for the antibody of interest and a selection marker/s, and genes that enable expression of these. Selection marker systems commonly used in Chinese hamster ovary (CHO) cells – the cell line of choice in CLD – utilise glutamine synthetase (GS) and dihydrofolate reductase (DHFR). In this selection system, the GS is inhibited by methionine sulphoximine (MSX) to block glutamine formation and DHFR is inhibited by methotrexate (MTX), which inhibits RNA and DNA synthesis. Selection of cells with higher introduced gene copy number takes place at the highest concentration of the drug that allows cell survival.
A more robust selection process is via selection marker attenuation, where a weaker promoter for the GS selection marker gene is used, lowering its expression. CHO cell lines expressing GS from an attenuated promoter show higher IgG-specific productivity and lower glutamine production than cells with selection expression under a stronger promoter.4
For gene expression the promoters used are often viral or endogenous. However, their unpredictability makes these less ideal for biopharmaceutical manufacturing. Synthetic promoters offer an alternative solution by matching the host cells’ transcription machinery to enhance expression, and are more predictable.
Variable cell growth rates
Single cell subcloning can be used to reduce variation of large heterogenous cell populations that are generated during host cell line engineering. However, generating a cell line from a single progenitor cell is one of the biggest challenges during CLD as cells grow in response to growth factors and nutrients, usually provided by neighbouring cells through autocrine signalling. The growth of single cells is therefore slow. Additionally, high-producing cells have high metabolic requirements for protein production, therefore, cell growth is hindered even further.
To enhance single-cell survival and support colony formation, optimising the culture medium for the specific cell line is critical. In CLD, the use of animal-free media is essential to avoid contamination with transmissible spongiform encephalopathies. However, single cells often struggle in serum-free conditions, so plant-based alternatives have been developed to provide necessary nutrients. To address the lack of autocrine signals, conditioned media can be obtained by culturing host cells in parallel, concentrating the medium and using it as a supplement. However, this strategy produces medium that is undefined. To address this, defined autocrine factors as well as paracrine factors that support cell growth have since been identified, which can be used to supplement media.5
Traditionally, culture medium optimisation involved adjusting one factor at a time: a time-consuming process. Faster alternatives include media blending – which tests various pre-formulated media mixes to identify the best combination for cell proliferation and productivity – and spent-medium analysis, comparing used and fresh medium to determine essential components and their optimal ratios. While medium optimisation can be labour-intensive, high-throughput, droplet-based microfluidic technologies now allow for numerous cloning experiments to be conducted quickly, accelerating the discovery of optimal medium compositions.
Cloning bottlenecks and maintaining viability during automation
As manual cloning techniques – such as limiting dilution – are slow, labour intensive, prone to contamination and expensive, more sophisticated, high-throughput and automated technologies are increasingly being adopted within the industry. Flow cytometry or fluorescence-activated cell sorting (FACS) has been used widely for cell selection, however, when used for high throughput screening, cells can experience significant shear stress, which can reduce cell viability.
In droplet-based microfluidics technologies, the encapsulation of cells within their preferred medium protects cells from stress or damage while the oil phase carrier fluid enables gas exchange, both supporting the encapsulated cells’ viability. Droplet-based microfluidic approaches therefore permit high-throughput processing without compromising viability.
Automated solutions that replicate specific steps of limiting dilution, such as cell printing, colony picking and cell-in-well imaging, offer incremental improvements by reducing time, labour and contamination risks. However, droplet-based microfluidics presents a more comprehensive approach by enabling full-process automation rather than individual steps. For example, while colony pickers can screen 10,000 cells in approximately three weeks, automated integrated platforms can screen up to 40 million cells in a matter of hours, significantly accelerating the selection process for high-yielding clones.
“When combined with a fluorescence detection system, this approach can be applied in droplet-based systems to automate the screening of large, heterogeneous cell populations, allowing for the identification and selection of individual cells with specific phenotypes or features of interest while preserving their viability”
Droplet microfluidics: advancing capabilities
Automated, droplet-based approaches have demonstrated a solution to address the challenges faced during CLD processes. By integrating the entire process, millions of cells can be screened to identify and select optimal clones for further characterisation. Fluorescence-based detection remains the predominant method within these systems, allowing single-cell assessment of key traits like target protein productivity and cell viability. Typically, these characteristics are analysed sequentially, but recent advancements in multiplexing capabilities have enabled more sophisticated, parallel assessments. These advances use additional lasers and photomultipliers to allow simultaneous evaluation of multiple cell characteristics, enabling the analysis of complex cellular profiles. This multiplexed approach increases the speed and efficiency of cell selection, facilitating the rapid identification of top-performing clones that meet production and viability standards.
Conclusion
Challenges in CLD are exacerbated when processes are scaled to meet the demands of biopharmaceutical development and manufacturing. Understanding these hurdles is key to effectively identifying and implementing opportunities for innovation, which is strengthened through collaboration and discussion between technology and biopharma companies. Combining the expertise from each organisation can be instrumental to the development and integration of technology to deliver transformative solutions.
Advances in single cell analysis, including the development of droplet-based microfluidic systems, play a critical role in CLD process improvement. This has been demonstrated through strategies that ensure the most suitable clones are selected early on, using targeted integration to reduce clonal variation during cell line engineering, and approaches to support cell viability and proliferation. Leveraging high-throughput, integrated droplet-based systems maximises the efficiency of this entire process, to support CLD optimisation and the faster identification of the best cells – easily.
References
- Visit: fortunebusinessinsights.com/biopharmaceuticalsmarket-106928
- Zboray K et al (2015), ‘Heterologous protein production using euchromatin-containing expression vectors in mammalian cells’, Nucleic Acids Research, 43(16), e102
- Chi X et al (2019), ‘A system for site-specific integration of transgenes in mammalian cells’, PLoS One, 14(7), e0219842
- Sacco S A et al (2022), ‘Attenuation of glutamine synthetase selection marker improves product titer and reduces glutamine overflow in Chinese hamster ovary cells’, Biotechnology and Bioengineering, 119(7), pp1712-1727
- Lim U M et al (2013), ‘Identification of Autocrine Growth Factors Secreted by CHO Cells for Applications in SingleCell Cloning Media’, Journal of Proteome Research, 12(7), pp3496-3510

Dr Maryam Ahmadi, director of Science at Sphere Fluidics, brings over 17 years of comprehensive experience in both industry and academia to her role. With a decade-long tenure at a contract research organisation (CRO), Maryam honed her skills in managing diverse projects. Notably, as the former director of Bioassay at Abzena, she led a team specialising in bespoke Immunology and Bioassay for external clients. Her responsibilities included the development of service lines crucial for testing and de-risking therapeutics, such as PK studies, MHC-associated peptide proteomics (MAPPs), and Cytokine Release Assay for therapeutic antibodies and antibody-drug conjugates. Maryam’s expertise spans Immunology and Tumour Biology, underscored by her proficiency in designing and overseeing tailored R&D initiatives. Her academic journey includes a postdoctoral research position at University College London (UCL), UK, where she delved into tumour-specific immune responses. She holds a PhD in Tumour Immunology from the University of Bristol, UK.