
Analytics
Innovations in pharmaceutical Analytics Including Microscopy, Chromatography, Spectroscopy, Separation Technologies, Flow Cytometry, Microarrays and RAMAN Imaging

1-3 July 2025, Manchester, UK

Bispecific antibodies are revolutionising biotherapeutic development, offering enhanced efficacy by targeting two antigens simultaneously. However, their structural complexity presents significant purification challenges. Advances in mixed-mode chromatography and scalable purification strategies are optimising production, improving yield, purity and cost efficiency, while ensuring a seamless transition from research to commercial manufacturing

Ruizhi Wang at Abselion talks to IPT about how non-optical sensor technologies are unlocking critical process data at the bench and bioreactor

One of the biggest events of its kind in Europe, mmc2025 (incorporating EMAG 2025) will bring you the very best in microscopy, imaging and cytometry from across the globe.

Society recognises outstanding achievements in microscopy, imaging and flow cytometry

Recent advancements in MALDI mass spectrometry imaging are transforming disease understanding and treatment. This innovation promises rapid diagnostics and personalised medicine, with reliable imaging within five years
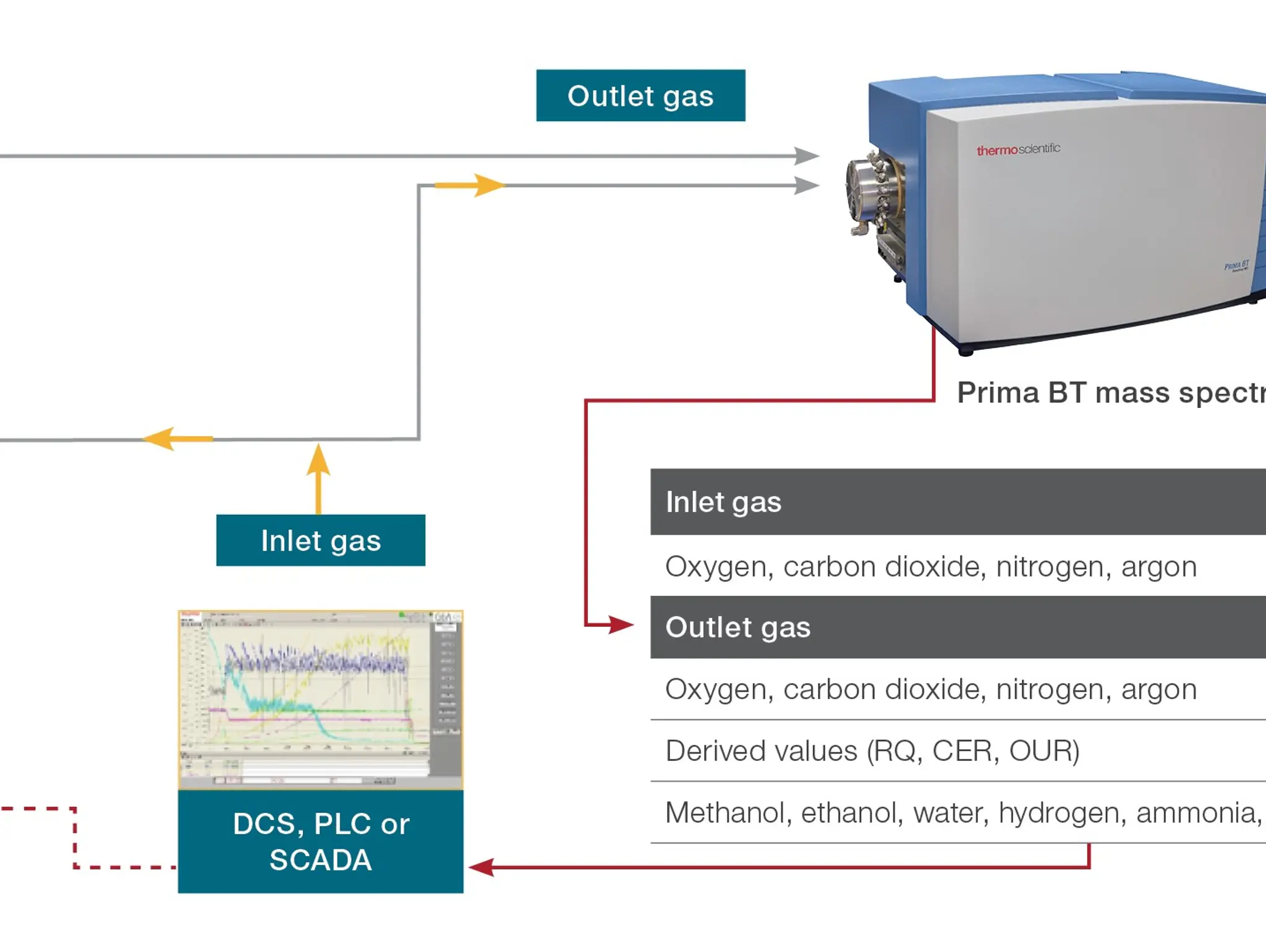
Thermo Fisher Scientific discusses the role of process analytical technology in addressing the process challenges concerning fermentation and cell culture in biotechnology and enhancing quality and speed production

IPT talks to Yama Abassi at Agilent about immunology and cell analysis and how these will develop in the future

IPT caught up with Peter O’Toole, president of the Royal Microscopical Society, to hear how his first few months as president have gone, his plans for the Society and how the field of microscopy might develop over the next few years

Www.mmc-series.org.uk
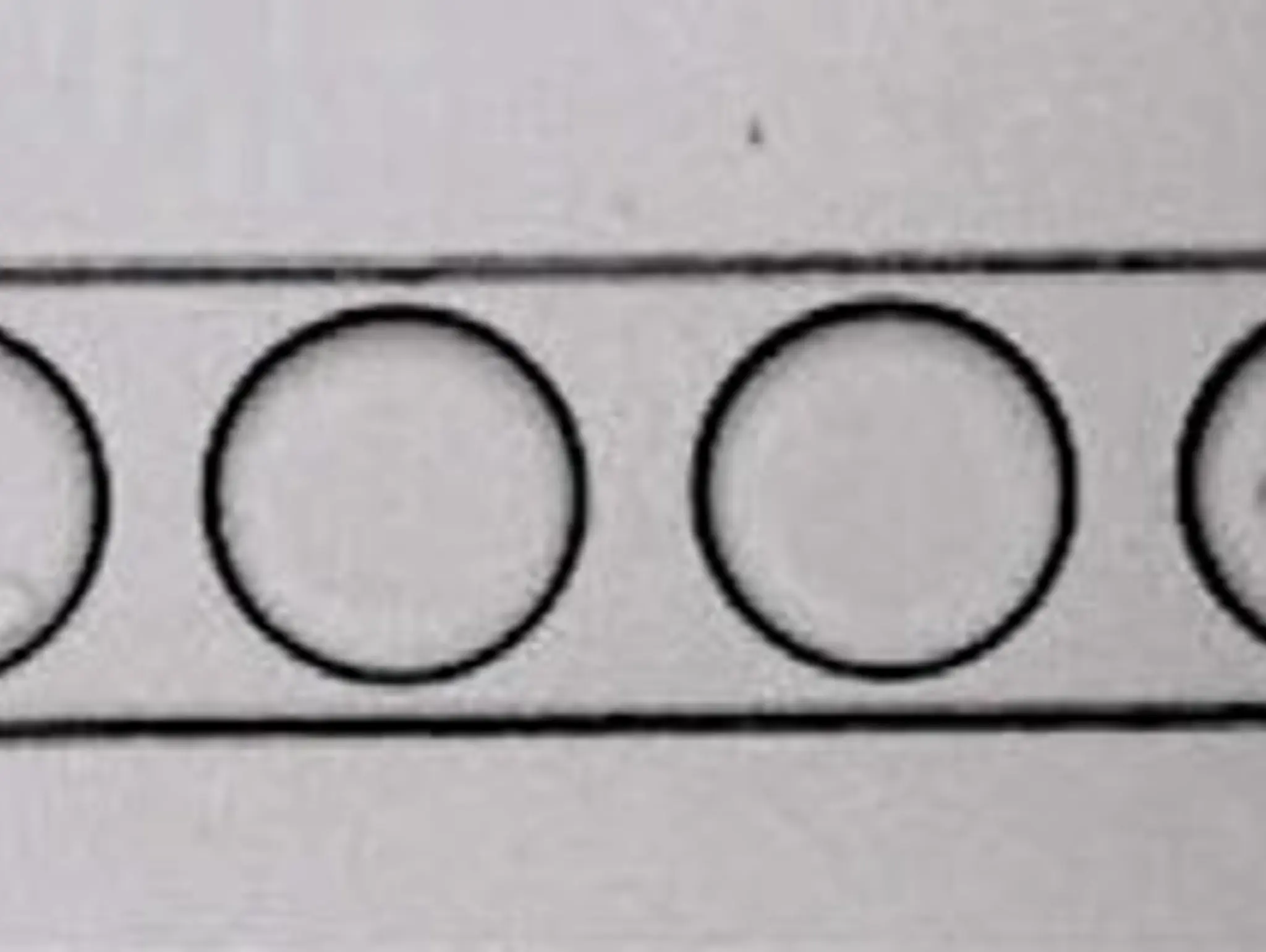
Droplet-based single-cell analysis systems provide an invaluable tool to accelerate research and development. Recent scientific advances enhance the applicability of these droplet-based systems to address the age-old problem of characterising, understanding and selecting cells

Flow cytometry is a powerful technology in the emerging cell and gene therapies (CGT) field and plays a significant role in various stages in the adoptive cell therapies, but what are its advantages and limitations?