Chromatography
Overcoming challenges in bispecific antibody production with mixed-mode chromatography and scalable purification solutions
Bispecific antibodies are revolutionising biotherapeutic development, offering enhanced efficacy by targeting two antigens simultaneously. However, their structural complexity presents significant purification challenges. Advances in mixed-mode chromatography and scalable purification strategies are optimising production, improving yield, purity and cost efficiency, while ensuring a seamless transition from research to commercial manufacturing
Daniel M Yoshikawa at Bio-Rad Laboratories
Bispecific antibodies (bsAbs) represent a rapidly expanding class of biotherapeutics, mainly due to their ability to simultaneously recognise and target two different antigens, enhancing drug efficacy and broadening therapeutic applications. Their clinical potential has led to the development of over 50 recombinant formats, each exhibiting unique structural and functional characteristics with distinctive therapeutic benefits.11 These formats are broadly categorised as asymmetric, symmetric and fragment-based bsAbs, reflecting their diverse structures. While advancements in upstream processing have significantly improved the yield and development of these different antibody formats, challenges associated with increased impurity levels and the inherent characteristics of bsAbs contribute to greater product heterogeneity, complicating downstream purification and processing.2,3
As antibody-based therapeutics continue to dominate the biopharmaceutical pipeline, there is a pressing need for robust purification strategies that balance efficiency, scalability and cost-effectiveness. Column chromatography is extensively used for antibody purification due to its high selectivity and versatility, though its efficiency depends on the selection of appropriate resins for each stage of the process. Since different resins operate within distinct technical parameters, purification has traditionally involved a three-step process, consisting of capture, intermediate purification and final polishing. Each stage must be tailored to the specific properties of the target antibody and the nature of the impurities that need to be removed, which may include host cell proteins (HCPs), DNA, endotoxins, viruses and aggregates. To ensure efficient bsAb production, purification strategies must achieve a balance between speed, yield, purity and cost-effectiveness while maintaining product integrity and safety. Employing chromatography tools with high specificity capabilities that can reliably overcome challenges posed by bsAbs in both smalland large-scale production settings is essential in ensuring their advancement as biotherapeutic agents.
Limitations of traditional purification strategies
Given the structural similarities between the two antibody classes, bsAb purification workflows are often adapted from established monoclonal antibody (mAb) purification methods.3 Traditional mAb purification relies on distinct chromatographic interactions between the stationary resin and mobile phase, with protein A affinity chromatography typically utilised during the initial capture phase. Protein A offers high specificity and produces a relatively pure product, however it has notable limitations including high costs and an inability to distinguish between functional and aggregated antibodies. Additionally, protein A can leach into the purified sample during acidic elution, requiring additional polishing steps, often involving ion exchange chromatography (IEX) followed by hydrophobic interaction chromatography (HIC) to remove residual impurities. As IEX resins have been used to capture mAbs as an alternative to protein A resins, they may also be screened for bispecific capture workflows.4 IEX-based approaches may be more cost effective and they inherently eliminate the need to remove any leached protein A.
While mAb-based protocols provide a useful starting point, further optimisation is often required due to bsAbs’ distinct physicochemical properties and the presence of unique byproducts that complicate purification. A key challenge in bsAb purification is the presence of product-related impurities, which include half antibodies that fail to form a full bispecific structure, and antibody fragments that closely resemble the desired product in size and physiochemical characteristics. The similarities between these impurities and fully assembled bsAbs makes separation particularly difficult, often reducing overall purification efficiency.

Figure 1: Three bispecific model molecules, including two asymmetric molecules with heterodimeric knob-into-hole Fc and one symmetric molecule with homodimeric wild-type Fc (CC BY 4)
Additionally, bsAbs are intrinsically more prone to aggregation compared to their parental mAbs, resulting in reduced stability and increased purification complexity.5 Adding to this issue is ‘chromatography-induced aggregation’, which has been observed during both protein A and cation exchange chromatography (CEX) for capture, particularly when bsAbs are subjected to high loading conditions.5,6 Although lowering column loading can reduce aggregation, it also decreases overall productivity, creating a major bottleneck in large-scale bsAb manufacturing.
As a result, while protein A and IEX chromatography remain key tools for bsAb capture purification, additional process optimisation or alternative purification strategies may be necessary to maintain both efficiency and product stability. Developing purification workflows that account for the distinct structural and aggregation tendencies of bsAbs is critical in achieving high-purity biotherapeutics at a commercial scale.
Effectiveness of mixed-mode media for bsAb purification
To address the limitations of single-mode purification methods, mixed-mode chromatography (MMC) has emerged as a promising alternative for bsAb purification. By combining multiple separation principles, MMC enhances purification efficiency and can serve as a complementary step in existing workflows. One example of this approach involves ceramic hydroxyapatite mixed-mode medium, a spherical microporous form of hydroxyapatite exhibiting both calcium metal affinity via its C-sites and CEX capabilities.7 Ceramic hydroxyapatite excels in removing HCPs, DNA and other process-related impurities to nearly undetectable levels due to its unique selectivity. This capability allows it to resolve mixtures that appear homogeneous, achieving a level of purity that other chromatography resins cannot match.
A recent study by Ingavat et al evaluated ceramic hydroxyapatite for use in a polishing step for bsAb purification, comparing its performance to traditional CEX chromatography following protein A capture.2 Three model bsAbs, each with distinct formats, molecular sizes and domain compositions, were assessed for purity and aggregate clearance (Figure 1). The results of this study demonstrate that the ceramic hydroxyapatite was effective for both asymmetric and symmetric IgG-like bsAbs, with each format exhibiting different binding and elution mechanisms (Figure 2). For both asymmetric and symmetric IgG-like bsAb eluates, ceramic hydroxyapatite polishing achieved at least 97% product purity while delivering superior aggregate clearance, compared to CEX.2 High molecular weight (HMW) impurities were significantly reduced to 0.5%, and process-related impurities were effectively minimised.2 Notably, the ceramic hydroxyapatite reduced aggregate formation during purification, particularly when NaCl was used as an eluent, whereas CEX chromatography resulted in significantly higher aggregate content, generating eight times more HMW impurities.2 Additionally, the ceramic hydroxyapatite demonstrated effective clearance of low molecular weight (LMW) impurities through post-load-wash optimisation, resulting in an additional 48% reduction in LMW contaminants.2
“ Developing purification workflows that account for the distinct structural and aggregation tendencies of bsAbs is critical in achieving high-purity biotherapeutics at a commercial scale ”
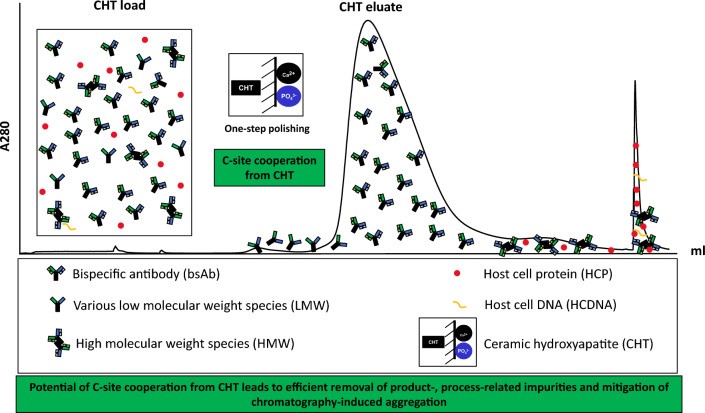
Figure 2: Ceramic hydroxyapatite is highly effective as a polishing step for bsAb purification delivering exceptional product purity and aggregation clearance following elution (CC BY 4)
The authors also proposed that the presence of calcium ions at the C-sites, which may increase hydrophobicity in the solution, could be the reason behind ceramic hydroxyapatite superior impurity removal and aggregation mitigation. This change in solution characteristics could enhance hydrophobic interactions with proteins, thereby diminishing intermolecular hydrophobic interactions between protein molecules and reducing aggregation – the effectiveness of which depends on bsAb composition and size.
The ability of ceramic hydroxyapatite to minimise process-induced aggregates further underscores its suitability for commercial-scale bsAb purification, ensuring high productivity and product stability.
Similarly, a study by Song et al further explored the effectiveness of ion exchange and mixed-mode resins in removing process byproducts during bsAb purification.8 Mixed-mode anion exchange chromatography was found to be effective in the polishing step of bsAbs with strong hydrophobicity, however ceramic hydroxyapatite chromatography demonstrated superior separation performance compared to CEX and conventional mixed-mode resins. Process scalability was confirmed in a 200L pilot batch, obtaining final products with 99.0% purity and a yield of 80.7%.8 These findings highlight the potential of ceramic hydroxyapatite chromatography for improving efficiency in large-scale bsAb purification, offering a cost-effective solution for downstream processing.
Advancements in high-specificity chromatography media and optimised purification workflows have significantly improved separation efficiency by enhancing impurity removal and minimising aggregation. Ensuring that these purification methods are scalable is crucial for transitioning from research-scale production to commercial manufacturing, allowing for greater productivity without compromising product quality.
Enhancing biotherapeutic manufacturing with scalable chromatography solutions
As MMC continues to gain traction, the adoption of prepacked multimodal chromatography columns has played a crucial role in optimising biotherapeutic manufacturing. Prepacked columns streamline downstream processing by reducing material waste, eliminating labour-intensive in-house column packing, and removing the need for performance testing and validation. These benefits contribute to improved productivity and enhanced cost efficiency, which has the potential to reduce overall manufacturing costs.
Additionally, ready-to-use prepacked columns manufactured with single lot resins ensure consistent performance, eliminating the need for specialised equipment and expertise, while minimising contamination risks commonly associated with manual packing.
Commonly used for process development and small-scale studies, prepacked columns are becoming more widely implemented in large-scale biomanufacturing operations that adhere to good manufacturing practice standards. The successful scalability of these columns, from small bench-scale formats to large process-scale columns, demonstrates their versatility in supporting all production needs.9 Suppliers offering prepacked columns provide solutions for seamless scale-up, delivering columns in a range of sizes prepacked with different resins, along with detailed documentation and hardware compatibility. Ensuring compliance with stringent specifications allows for straightforward integration into high-throughput and large-scale purification processes.
Conclusion
The growing global demand to address complex diseases with next-generation antibody therapeutics, such as bsAbs, underscores the need for efficient, scalable and high-specificity purification strategies. While traditional mAb purification methods provide a foundation, the unique structural and physicochemical properties of bsAbs demand tailored approaches to ensure high yield, purity and stability. Challenges such as product-related impurities, aggregation and chromatography-induced instability necessitate the adoption of advanced purification techniques, including MMC resins like ceramic hydroxyapatite.
Additionally, the integration of process-scale prepacked chromatography columns is streamlining downstream processing, reducing operational costs and facilitating seamless scale-up from research to commercial manufacturing. By leveraging these innovative solutions, the biopharmaceutical industry can overcome bsAb purification challenges, ensuring cost-effective, high-quality production workflows.
References:
1. Visit: sciencedirect.com/science/article/abs/pii/S0021967325000718
2. Ingavat N et al (2023), ‘Harnessing ceramic hydroxyapatite as an effective polishing strategy to remove product-and process-related impurities in bispecific antibody purification’, Bioresour Bioprocess, 10(1), 93
3. Chen S W et al (2021), ‘Current trends and challenges in the downstream purification of bispecific antibodies’, Antib Ther, 4:2, 73-88
4. Ng P K et al (2012), ‘pH-based cation exchange chromatography in the capture and elution of monoclonal antibodies,’JSep Sci, 35(1), 29-35
5. Chen S W et al (2022), ‘Excellent removal of knob-intohole bispecific antibody byproducts and impurities ina single-capture chromatography’, Bioresour Bioprocess, 9, 72
6. Chen S W et al (2022), ‘Effective flow-through polishing strategies for knob-into-hole bispecific antibodies’, Bioresour Bioprocess, 9, 98
7. Cummings L J et al (2014), ‘Monoclonal antibody purification by ceramic hydroxyapatite chromatography’, Methods Mol Biol, 1131, 241-251
8. Song M et al (2023), ‘Separation of bispecific antibody related impurities with mixed-mode chromatography’, Process Biochemistry, 132, 110-120

Dr Daniel M Yoshikawa is a senior global product manager with the Process Chromatography Business at Bio-Rad. He received a BS degree from the University of California, Davis, US, and his PhD degree in Pharmacology at the University of Rochester, New York, US. He is responsible for bringing Bio-Rad’s mixed-mode process resins to market. He takes great pride in connecting with life sciences researchers to stay abreast of industry needs.