The Automated Lab
Automated labs: the future of drug R&D
IPT talks to David Fuller at Artificial Inc about how automated labs are revolutionising the pharma R&D space by connecting disparate systems, easing the burden of manual tasks on workers, and speeding up time-to-market with tools such as artificial intelligence

IPT: What are the main differences between a classic lab set-up compared to an automated lab set-up?
David Fuller (DF): The primary differences between classic and automated lab set-ups revolve around the level of automation, digitisation and the processes they employ. Classic lab set-ups are characterised by manual operations where lab staff are heavily involved in every step of the process. This includes tasks such as experiment set-up, data collection and analysis, often using paper or semi-digital systems. As a result, these labs typically have lower throughput, are more prone to human error and may lack centralised digital records. Additionally, classic labs generally rely on separate, isolated systems for data management, which can lead to inefficiencies in data retrieval and analysis.
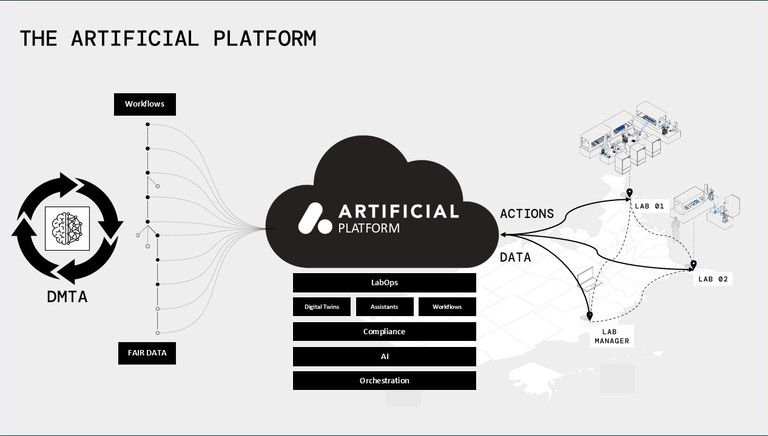
In contrast, automated labs are built on the principles of digitisation and automation, combining these elements to enhance efficiency, repeatability and reliability. Automated labs use advanced systems, such as robotics, AI-driven decision-making and data processing tools, to streamline experimental procedures and data collection. With automation, labs can handle high-throughput tasks, running multiple experiments simultaneously and generating large volumes of data. These systems ensure consistency and reduce human error, allowing faster experimentation and decision-making.
Automated labs also rely on integrated digital platforms that provide a centralised database for all recorded data, making accessing, analysing and sharing findings easier. Ultimately, the shift to automation in lab set-ups allows for more efficient workflows, lowers the cost per data point, and enables results that are higher quality and repeatable.
IPT: In what ways will automated labs work alongside lab staff to maintain productivity while integrating new systems?
DF: Automated labs are designed to complement lab staff. While automation introduces new technologies and systems into the lab, integrating these systems allows lab personnel to maintain or enhance productivity. The key to this integration is the digitisation of lab processes. Even if specific tasks remain manual, they are still documented and managed in a digital format, creating a smooth interface between human and machine-driven workflows. Automation enables staff to focus on more advanced, higher-level tasks. For instance, staff can engage in data analysis, troubleshooting and strategic decision-making, while the automation handles repetitive, time-consuming tasks like sample preparation or running tests. Artificial intelligence (AI) systems can guide staff through digital processes, whether these processes are performed manually or automatically – ensuring accuracy and efficiency in task execution. Moreover, automated systems are adaptable, enabling staff to be able to intervene, optimise workflows or make adjustments based on real-time data, when needed.
IPT: Can real-time monitoring through connected systems reduce bottlenecks in pharma manufacturing?
DF: Yes, real-time monitoring through connected systems can significantly reduce bottlenecks in pharma manufacturing. Real-time monitoring allows labs to track each step of the process as it occurs, providing visibility into any inefficiencies or delays. In a traditional lab set-up, bottlenecks often go unnoticed until they cause significant disruptions. However, with automated systems and connected monitoring, any signs of delays or issues are immediately detected, and tasks can be rerouted or adjusted dynamically to available resources.
In pharma manufacturing, where the production of drugs must adhere to strict timelines and quality controls, even small delays can have cascading effects on the entire production process. Real-time monitoring systems enable labs to proactively identify and address bottlenecks before they escalate. For example, if a machine or step in the process is running behind schedule, the system can redirect tasks to other machines or adjust the timeline to maintain overall workflow. This real-time orchestration ensures that resources are utilised efficiently and tasks are completed in the most optimal sequence, reducing downtime and maximising throughput.
Moreover, the data collected through real-time monitoring can be used to identify patterns in bottlenecks, allowing for predictive analytics to prevent future delays. This proactive approach to managing manufacturing processes improves overall efficiency, reduces production costs, and ensures that drug development and manufacturing timelines remain on track.
IPT: How effective are cloud-based storage solutions for managing and utilising captured lab data?
DF: Cloud storage has emerged as the leading standard due to its many advantages, particularly for research labs that handle large volumes of data. A significant benefit of cloud storage is its global accessibility. Researchers, lab personnel and decision-makers can access data from any location, which enhances remote work and collaboration. This feature is especially beneficial for labs within larger organisations or networks, as it allows teams to share and work with data in real time, regardless of their physical location.
Cloud-based storage effectively addresses the issue of data silos – a common challenge in traditional lab environments. In many conventional labs, data is stored in isolated systems, making it difficult to integrate or comprehensively analyse. With cloud storage, data from various departments, machines and experiments can be centralised in one easily accessible location. This enhances data visibility, ensuring that all pertinent information is readily available for analysis and decision-making. Additionally, cloud solutions provide greater scalability and flexibility than traditional on-premises storage. Labs can expand their storage capacity as needed without worrying about physical hardware limitations.

Furthermore, cloud-based systems often have built-in data security features, ensuring that sensitive research data is protected from unauthorised access or loss. Cloud storage also enables advanced analytics and AI tools to analyse lab data more effectively.
Labs can apply machine learning models and predictive algorithms to identify trends, optimise processes and make data-driven decisions with data stored in the cloud. Integrating cloud storage with automated lab systems enhances the overall efficiency of lab operations, and ensures that data is managed and utilised to its full potential.
IPT: How could the integration of automatic lab set-ups revolutionise pharma R&D over the next five years?
DF: The integration of automated lab set-ups is poised to revolutionise pharmaceutical R&D in several ways over the next five years. One of the most significant impacts of automation in pharma R&D will be the rise of high-throughput experimental labs. These labs will be able to conduct a much larger number of experiments simultaneously, generating vast amounts of data that can be used to accelerate drug discovery.
Automation will enable the pharmaceutical industry to explore more compounds, test more variables and gain deeper insights into the effects of potential drugs more quickly than ever before. With automation handling many of the repetitive and time-consuming tasks, researchers can focus on higher-level analysis and decision-making.
AI-driven systems will play a critical role in interpreting the massive volumes of data generated by automated experiments. AI can help identify patterns, predict outcomes and suggest new avenues for research, speeding up and reducing the cost of the drug discovery process. Furthermore, integrating automation will enhance the scalability of drug development. As automation allows for more experiments to be conducted simultaneously, pharmaceutical companies can quickly adapt their research. This level of agility is particularly important in drug discovery, where the ability to pivot quickly can lead to breakthroughs. Adopting automated lab set-ups in pharma R&D will accelerate the pace of discovery, improve the efficiency of drug testing and potentially lead to the development of more effective treatments in a shorter time frame. This will ultimately have a transformative impact on the pharmaceutical industry, making drug development more efficient, cost-effective and responsive to the needs of patients.

David Fuller is the co-founder and CEO of Artificial Inc. David has over 25 years of experience in industrial automation and software platforms. He received his BS in Computer Engineering from Texas A&M University, US, and in 2011 was recognised by the Texas Academy of Medicine & Engineering for Technology Innovation. He holds 42 patents. He is driven by a love of technology that has a clear, positive impact on society.