Manufacturing: Biomanufacturing Operations
Cell processing platforms in biologics therapeutics manufacturing
Julien Muzard at Entegris speaks to IPT about how innovations in downstream cell processing platforms will improve biologics therapeutics manufacturing, and the impact artificial intelligence will have on the sector
IPT: Why is risk management so important in novel biologics therapeutics manufacturing?
Julien Muzard (JM): Risk management is of critical importance in contemporary biomanufacturing to minimise process-related impurities, meet quality and regulatory expectations, and, ultimately, ensure product safety in patients. The risk management framework spans across all phases, from R&D through scale-up and commercial development, enhancing the robustness of the manufacturing process. Globally, risk minimalisation involves a tight control of every step, from supply of raw materials and components up to final actions of the manufacturing operations and products’ life in patients, while offering full traceability – ensuring quality and latest regulatory compliance. As an example, a contamination risk due to unwanted, uncharacterised particles at the nanosize level could be dramatically reduced to an extremely low defect when working with premium materials and carefully designed operations. The advances in cell and gene therapies (CGTs) and molecular biology, programming with DNA, genomics, engineering and semiconductors, is revolutionising the discovery and manufacturing of medicine today.
A real challenge is applying this approach to the shifting landscape, from blockbuster drugs and traditional recombinant/first generation biologics such as mAb, to antibody fragments and recombinant proteins utilised in personalised treatments – driving the need for innovative processing strategies. The challenges of manufacturing in small batches can be addressed with carefully designed, automated processes to eliminate human intervention and associated variabilities. This is one of the multiple strategies for achieving success, and is critical to driving efficiencies to help ensure the proliferation of modern therapies as they face pressures such as high treatment costs due to complex processing and small batch sizes associated with individualised therapies. The concept of one blockbuster dose for everyone is changing to a more personalised medicine to solve specific patient issues. The next question is how do we accelerate this?
IPT: How can innovations in downstream cells processing platforms improve current processes?
JM: Biomanufacturing operations remain based on very mature scientific principles and physical procedures which were first discovered decades ago (eg, filtration, separation and chromatography). Innovations in downstream processing platforms, using ready-to-use systems, particle removal techniques, high-throughput purification screening, and hardware and advanced modelling, can enhance efficiency, reduce overall costs and streamline purification, ultimately addressing bottlenecks and improving the overall productivity of biopharmaceutical manufacturing.
One of the critical parameters to consider is the operational and running costs of the current upstream and downstream processes. This breaks down into several categories: set up; development; materials; and labour. These can be impacted by utilising easy-to-implement processes with low upfront costs, tight monitoring tools and controls, choosing phase-appropriate materials at all stages, and implementing systems that are consistent and repeatable to avoid errors.
The largest costs are often not manufacturing costs, but the costs of poor quality, poor performance, or reworking/troubleshooting in any process. Breaking down the hardware, the consumables, the packaging and the labour with innovative solutions would ultimately contribute to better access to innovative personalised medicine from a socioeconomical perspective. Innovation in those sub-steps would also accelerate time to market, lowering waiting time for patients to have access to the cell-based medicine they need.
Digitalisation and improved analytics of the whole supply chain is already enabling greater visibility and agility. By integrating new innovative solutions into cells processing tools, we are able to dynamically reset positions as conditions change to innovations and anticipate the market needs.
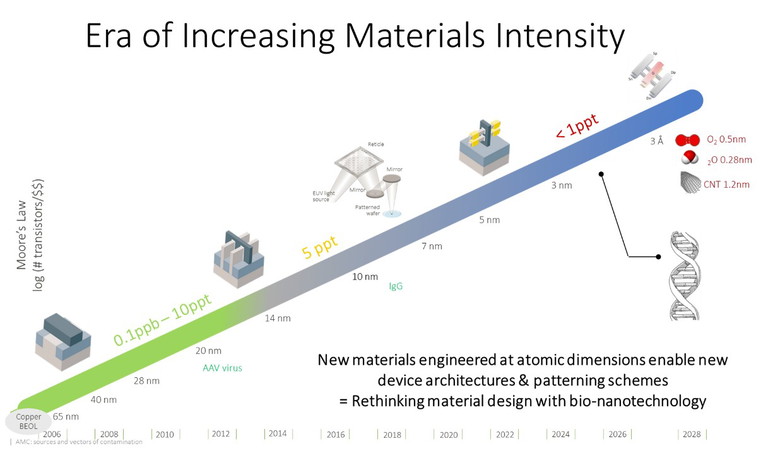
Figure 1: Fusion semiconductor and life sciences: era of increasing materials density
IPT: How much of an impact will artificial intelligence and machine learning have on risk management applications?
JM: Digital designs, artificial intelligence and machine learning (AI/ML), and simulations tools are beginning to be applied to make processes faster while streamlining risk management with an even more robust framework. There are already advances in synthetic biology with AI de novo protein engineering and computer-based cells design, vaccines and biochemical circuits, for example. Protein, virus and vaccine engineering has gone fully digital now. Biomolecular interactions can be predicted, 3D novel proteins designed computationally, and large sets of genomic DNA assembled to synthesise viruses and organisms from scratch. This is moving the science to a field which used to be called computational biology, or bioinformatics, to a system biology, as everything in the lab across scales get connected.
The other top areas where AI can benefit biomedical and healthcare outcomes most are: identification of new targets or methodologies for early screening detection of diseases (especially genetic disorders); spotting trends in large data sets in mature manufacturing processes to identify places of failures or deviations; prediction of protein shape-function linked to clinical disorders; the deployment and training of new AI and ML tools at a large scale for faster monitoring related to the patient regimes and response to specified treatment.
AL and ML are transforming the way bioengineering is approached. In-depth analysis of complex data is aiding in the understanding of cellular and biological development and disease, development of new drugs and processes, and the enhancement of clinical outcomes. AI and ML can significantly enhance data analysis, predictive modelling and decision-making processes, ultimately contributing to more effective risk identification, assessment and mitigation. By leveraging these technologies, potential risks may be able to be identified quicker, more accurately, allowing staff to respond more efficiently to emerging threats and make more informed decisions.
“ In-depth analysis of complex data is aiding in the understanding of cellular and biological development and disease, development of new drugs and processes, and the enhancement of clinical outcomes ”
“ New supply chain management tools, such as those used by the semiconductor industry, can help biopharma companies forge strong relationships with suppliers and leverage technologies to provide real-time, end-to-end updates to predict and solve potential issues before they occur ”
One challenge is the efficient transfer of foundational models – the use of hugely diversified data obtained across scale and accessible within systems biology, single-cell multi-omics, multimodal imaging, biomanufacturing process data architecture and clinical advances – in digital systems based on large sets of data, and this is where AI and ML can help further. As the exploration and implementation of innovative technologies continues, it is essential to capitalise on the benefits and the implications of these advancements on risk management strategies.
Innovation in semiconductors and bioengineering/ synthetic biology will power the fourth industrial revolution, which will transform healthcare and more generally improve the way we work and live. New supply chain management tools, such as those used by the semiconductor industry, can help biopharma companies forge strong relationships with suppliers and leverage technologies to provide real-time, end-to-end updates to predict and solve potential issues before they occur. As a result, the semiconductor industry’s technological advances will play a key role in enabling the deployment and proliferation of personalised therapies for human health, and improve quality of life and life expectancy.
IPT: In what ways can ready-to-use systems be integrated into current biomanufacturing set-ups?
JM: It usually starts with integrating very simple components, such as targeting a specific step or sub-step within the bioprocess workflow like a bioreactor, centrifuge bags, analytical plug and play sensors, or cell banking/cryopreservation tools. However, the whole biomanufacturing process can be entirely covered using disposable technologies. This offers operational capability to compliantly deliver both traditional and advanced therapy medicines across many formats with ready-to-use systems. That integration into current biomanufacturing set-ups presents several opportunities for enhanced efficiency and productivity while reducing costs. Ways to achieve this integration are multiple, including starting from scratch, retrofitting existing plants or replicating integrated plants in another location across the globe. This streamlines all equipment installation by reducing the need for on-site assembly or custom configuration.
The integration allows for the quick adaptation of an existing facility infrastructure to accommodate off-the-shelf systems, or the replication of a whole plant on a multi-continent scale. The use of sterile, preselected, prepacked and validated components align with existing workflows and regulatory frameworks.

Furthermore, the approach minimises maintenance and systems calibration requirements for improved process control and digital twins. These strategies can facilitate the incorporation of ready-to-use systems into current biomanufacturing facilities, supporting ongoing process optimisation and future growth within small-scale R&D prototypes, to several thousand-litre bioreactors for commercial-scale operations.
IPT: How will the landscape of biomanufacturing change over the next five years?
JM: The biomanufacturing industry is expected to undergo significant changes over the next five years. With advancements in personalised genomics (eg, gene editing technologies such as CRISPR), continuous manufacturing tools may become more prominent for product development and process optimisation. Furthermore, the increasing prevalence of ready-to-use technologies in biomanufacturing may continue to gain momentum due to their flexibility, scalability, personalised packaging and reduced environmental impact. Additionally, emerging biologics are likely to play a larger role, with investments in R&D driving innovation in this area and hopefully reducing the overall cost for patients. In terms of regulatory environments, the industry is expected to see increased scrutiny on traceability, quality and good manufacturing practices regulations.
Lastly, the use of digital twins, AI and ML will become more prevalent in biomanufacturing, enabling improvements in process control and overall efficiency. To advance personalised medicine, deep learning architectures that incorporate large-scale biomedical/biomedical data sets and are deployable in the clinical setting or large-scale production have to be further adopted. There are unique technical constraints within current bioprocessing workflows, but combining these insights with engineering and product design will create high-performance, low-cost technologies that are relevant for the industry globally. However, traditional biologic production is a conservative industry, reticent to change, and clinical biotechnology is highly regulated, so the shift might be slow. It is expected the landscape will change within the personalised medicine market, making CGT products more affordable via the deployment of robotics and automation. With digitalisation transforming these processes, this has also the potential to drive increased adoption among more traditional therapies as well as disrupt other industries such as food and beverage, diagnostics, sustainable cosmetics and synthetic biology. Ultimately, it is important to characterise the unique technical constraints of those emerging markets and processes, then combine these insights with engineering and products designed to create high-performance, low-cost technologies inspired from semiconductors that are relevant for the industry globally. This will help to elucidate novel scientific and engineering knowledge, and will have a positive impact on patients.
Julien Muzard will offer his insights at INTERPHEX on 3 April in the panel discussion, ‘Advancing Biomanufacturing: From Ready-to-Use Systems to Data-Driven Risk Management and Optimization’

Julien Muzard is a field applications technologist Life Sciences – EMEA at Entegris. His career has been devoted on building next-generation platforms for precision bio-engineering and molecular electronics, focusing on designing new 3D architectures with programmable functions as sources of tools for research, diagnostics and therapeutics. He made a significant contribution to the creation of various genetically engineered composite particles and theragnostic molecules deployed in clinical stage and/or commercially. Julien studied diagnostic microbiology, toxinology, molecular biology, virology and immunotherapeutics, then obtained his PhD from Paris-Diderot, France. After postdoctoral fellowships, he continued his career at the Center for Molecular Innovation & Nanomedicine, University College Dublin, Ireland, and at Foundry, Berkeley Lab & Bio/Nano/Programmable Matter, Autodesk, California, US.