Peptide Therapeutics
Peptide therapeutics: reconstructing protein surfaces using constrained peptides
Proteins govern crucial biological processes, making protein-protein interactions vital for understanding and treating diseases, peptide-based mimics offer tailored therapeutic approaches, yet optimising their efficacy poses challenges
Peter Timmerman at Biosynth
Advances in genetics, bioinformatics and protein structural studies over the last few decades have allowed us to start unravelling the complex web of interactions that govern human health and disease. From enzymatic reactions to the structural integrity of cells, immune responses and signal transduction, proteins are the architects of life's fundamental processes, acting through specific interactions with their ligands.
Exploring protein-protein interactions (PPIs) is therefore critical to gaining insight into the molecular mechanisms underlying disease and other phenomena, in addition to developing novel therapeutic strategies. Molecules, such as peptide-based protein mimics that can specifically target protein binding sites with precision, offer a tailored approach for treating a myriad of diseases.1 These short chains of amino acids can be used to develop targeted drugs, therapeutic monoclonal antibodies (mAbs), or synthetic vaccines, targeting specimens that are considered difficult, or even ‘undruggable‘ using standard techniques (see Figure 1).2
Since the synthesis of the first therapeutic peptide, insulin, in 1921, more than 80 peptide drugs have been approved worldwide.3 They are used in a wide range of therapeutic areas such as urology, respiratory, pain, oncology, metabolic, cardiovascular and antimicrobial applications.4-8 To date, more than 170 peptides are in active clinical development, with many more in preclinical studies.3
Advances in structural biology, recombinant biologics and new synthetic and analytic technologies have significantly accelerated the development of peptide drugs. However, harnessing the full potential of peptide therapeutics is not without its challenges. A key challenge in peptide design and synthesis is to achieve a 3D structure that can perfectly mimic the target binding site. However, simple, linear peptides often lack conformational definition in solution, leading to compromised binding activity and reduced effectiveness. Consequently, post-synthesis modifications are often needed to refine peptides and precisely replicate the 3D structure of their target.
Constrained peptides are therapeutic protein mimetics that have been locked within a specific conformational structure to ensure therapeutic efficacy. In this article, this article will cover the benefits of peptide therapeutics, the importance of producing peptides in the suitable conformation, and how new technologies are streamlining this challenge.
Why peptide therapeutics?
Current drug discovery and development approaches are focused on three different types of molecules based on size: small molecules, peptides and biologics (see Figure 2). However, most investigational and approved drugs to date are either small molecules or macromolecular biologics, with antibodies, proteins and vaccines representing the predominant forms of approved biologic therapies.9 Until recently, aside from a small number of natural products, there has been much less progress in designing peptide drugs.
While small molecule drugs are relatively easy to synthesise, optimise and administer, this comes with the trade-off that they frequently possess off-target biological activity that may translate into unexpected biological effects and toxicity during clinical development.9 On the other hand, biologics, such as antibodies, are generated to be highly selective toward their desired target. However, they are also more complicated and expensive to manufacture, and can be immunogenic, so can only be administered by injection. In contrast, peptides represent the best of both worlds by being highly selective and efficacious and, simultaneously, less costly and immunogenic than biologics.3 However, naturally occurring peptides are often not directly suitable for use as convenient therapeutics because they have intrinsic weaknesses, including their low membrane permeability, poor oral bioavailability and short half-lives after administration.10 However, there are now strategies to optimise their natural sequences to overcome these challenges.
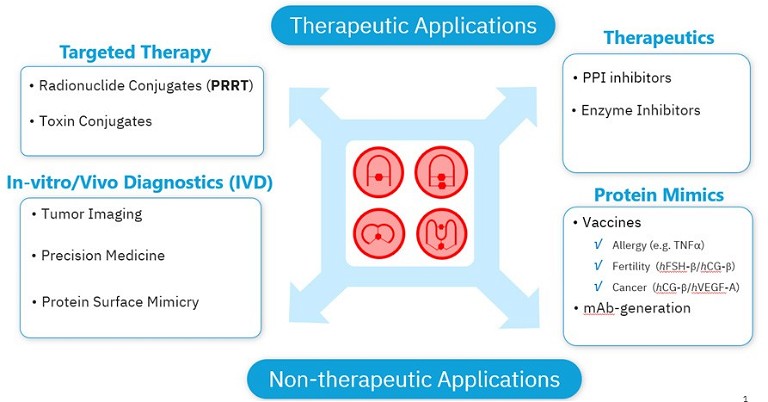
Figure 1: A broad range of applications of peptides
Constrained peptides: unlocking precision in molecular interactions
The rational design of peptides relies on bioinformatics technology, which uses computer-assisted tools to identify essential amino acids, often referred to as ‘hotspots‘, on the surface of protein binding sites.11
These hotspots can be a continuous fragment of the protein or dispersed residues that are distant in protein sequence, but brought into spatial proximity through protein folding. Once identified, peptide modulators are designed based on these hotspots. However, further peptide development and structure optimisation are required to improve their activity and physicochemical properties. For example, the study of the structure-activity relationship (SAR) can identify essential residues and optimise non-essential residues, while chemical modification of the sequence can help to stabilise the peptide secondary structure to enhance its efficacy and stability. Stabilising or restricting the peptide conformation to match the ligand-bound state significantly improves therapeutic efficacy, in addition to enhancing the peptide specificity and proteolytic stability.2 Termed constrained peptides, these 3D-structured molecules offer a precise and effective replication of the molecular interactions crucial for effective therapeutic outcomes.
In a 2016 study, researchers used this technology to produce a peptide therapeutic that can mimic the native structure of vascular endothelial growth factor (VEGF), particularly the bevacizumab binding site.12 Through vaccination with this peptide, the goal was to trigger an immune response in the host, leading to the production of antibodies that specifically target VEGF.
These induced antibodies would then function as therapeutic agents by binding to VEGF and neutralising its activity, thereby inhibiting tumour angiogenesis and growth. For peptide mimics like VEGF-trunc, it is important that they are exact mimics of the native protein that they are mimicking (in this case VEGF-A165), such that the immune system does not see the difference. As such, it was critical that the peptide vaccine accurately mimicked the binding site on VEGF.
Interestingly, researchers found a massive disparity between linear and constrained peptides that were targeting the binding site. Binding and competition studies showed that the main constrained peptide (‘peptide 1’) perfectly mimicked the complete bevacizumab binding site, including the hairpin loop (ß5–turn–ß6) and the structure-supporting ß2–ß2–ß3 loop that are crucial for binding. Vaccination with this peptide elicited high titres of cross-reactive antibodies with potent neutralising activity against VEGF. This was also shown to significantly inhibit the growth of melanoma in mice.
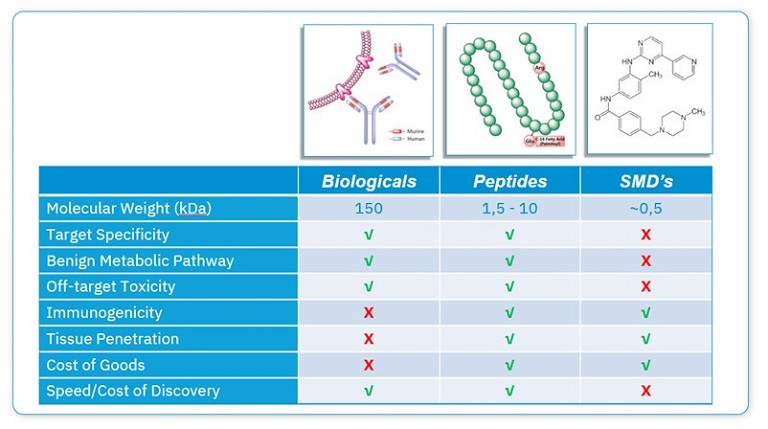
Figure 2: Comparison of small molecules, peptides and biologics and their key characteristics
In contrast, immunisation with peptides lacking these 3D structures only raised antisera devoid of neutralising activity for VEGF, and was ineffective in inhibiting tumour growth. This research significantly underscores the necessity of maintaining a native-like, secondary structure in peptides, especially for indirect therapeutic roles such as this.
In recent decades, several strategies have been developed to constrain a peptide into its bioactive target-bound conformation, including chemical and enzymatic cyclisation. Cyclisation is a common peptide modification technique that can include various strategies, such as head-to-tail, backbone-to-sidechain and sidechain-to-sidechain cyclisation.
Without being connected to other peptides, a single peptide sequence cannot form secondary structures such as ß-helixes and ß-sheets, which represent about 30-40% of all protein structures.
There is a variety of chemical methods available for the structural fixation of peptides into constrained peptides such as metathesis and CLICK chemistry. However, some of these methods require special protection/deprotection protocols, laborious experimental optimisation, or the addition of a catalyst, making the process more complex, time-consuming and inaccessible. New methods are being developed to streamline this process, ensuring the production of targeted, constrained peptides in a simplified manner.
Streamlining the discovery and optimisation of constrained peptides
In recent years, significant advancements have been achieved in developing novel cyclisation strategies for peptide macrocyclisation, including peptide stapling, enzymatic macrocyclisation and chemical ligation methods. These methodologies streamline the process of peptide macrocyclisation, removing the need for laborious protecting group strategies and enabling in vitro selection systems.
Peptide stapling is a well-established and evolving technique used to constrain peptides into a specific conformation, often an alpha helix, by introducing covalent bridges between specific amino acid side chains.13 These bridges, or staples, stabilise the secondary structure of peptides, enhancing their proteolytic stability, cell permeability and target binding affinity. This approach is particularly versatile with multiple strategies available including hydrocarbon staples, lactam bridges, hydrogen-bond surrogates and photoswitches. Each stapling method offers distinct advantages, influencing properties such as helicity, hydrophobicity and protease resistance.14 Peptide stapling is a powerful tool in drug discovery and development and is continuously advancing to enhance versatility, cost-effectiveness and accessibility in peptide engineering.
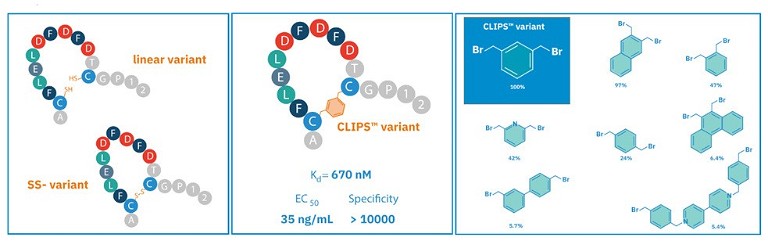
Figure 3. Different types of peptides, including a linear variant, a cyclised peptide with a disulphide bridge and a cyclised CLIPS variant bridging cysteines. The bromine-scaffolds can achieve a wide diversity of structures
Recently, enzymatic ligation strategies have also gained increased attention due to their inherent properties such as excellent regio- and chemoselectivity and the catalysis of reactions under mild conditions.15 In enzymatic macrocyclisation, specific enzymes, such as ligases or cyclases, are employed to facilitate the cyclisation process. These enzymes recognise and bind to the peptide substrate and then catalyse the formation of the peptide bond, resulting in the formation of a macrocyclic peptide. Enzymatic macrocyclisation offers several advantages, including high selectivity, efficiency and mild reaction conditions. By harnessing the catalytic power of enzymes, this approach enables the precise control of cyclisation reactions, leading to the production of structurally defined macrocyclic peptides with desired properties.
Finally, chemical ligation methods are powerful techniques used in peptide chemistry to join two or more peptide fragments together to form a larger peptide or protein.16 This approach is often used in conjunction with solid phase peptide synthesis (SPPS), enabling the stepwise assembly of peptides on a solid support by sequentially adding protected amino acids and coupling reagents.
Following the full assembly of the peptide chain on the solid support, it can be liberated from the resin and subjected to further modifications or conjugations using various chemical linkage techniques. There are many chemical linkage techniques used in conjunction with SPPS including native chemical ligation, click chemistry and peptide cyclicisation (see Figure 3). By using a scaffold, techniques such as this can construct bi- or tricylic structures and other scaffold designs with a much wider range of structural diversity and applications. This can identify bioactive molecules that can effectively bind to a much wider range of structurally diverse targets, including those sites considered undruggable with conventional small molecule drugs, such as flat surfaces engaged in PPIs.17
Applications and future prospects of constrained peptides
Peptide drug development has made great progress in the last decade thanks to growing interest in peptide-based therapeutics, advancements in peptide synthesis techniques and increasing research and development activity. Peptide drugs now account for a significant proportion of the pharmaceutical market, with worldwide sales projected to reach a staggering $75bn by 2028.18 This is likely driven by the rising prevalence of chronic diseases, such as cancer and diabetes, further fuelling the demand for constrained peptide drugs. There are now 80 therapeutic peptides on the market for the management of a wide range of diseases including diabetes, HIV and cancer, with more drugs currently in development. Recently, there has been rapid progress towards a peptide therapy that targets metastasis in cancer. Cell migration is a key feature of cancer metastasis and often indicates a more advanced disease state. One of the critical proteinases involved in cell migration is membrane-type 1 matrix metalloproteinase (MT1-MMP).19 Despite significant efforts dedicated to the development of interventions targeting MT1-MMP, success has remained elusive, primarily due to off-target effects leading to adverse side effects.
However, constrained peptides have helped to make progress in this area. Researchers have subsequently linked a toxin payload to the high-affinity bicyclic peptide to create a ‘bicycle drug conjugate (BDC)’. This compound, known as BT1718, has demonstrated a rapid ability to penetrate tumours and significant anti-tumour activity in preclinical studies.20 Because of its peptidic nature, BT1718 can be efficiently cleared through the kidney, minimising toxicity relating to payload-mediated gastrointestinal and liver effects. Cancer Research UK is currently supporting phase 2 trials of this drug in the hopes that it could reduce metastatic cancers in the future.
Peptides have undeniably played a crucial role in the advance of chemical and biological science, profoundly impacting the development of the modern pharmaceutical industry. Because of their unique biochemical characteristics and therapeutic potential, designer peptide drugs are now sought-after alternatives to small molecule therapeutics and a major focus for creating next-generation products. With continuous innovation and development strategies, including peptide cyclisation and crosslinking, peptides will continue to improve and fill the untapped gap between conventional small synthetic drugs and the more complex protein biologics.
References
- Venneti NM et al (2023), ‘Stretching Peptides Potential to Target Protein–Protein Interactions’, ACS Central Science, 9(4), 590–592.
- BozovičarKet al (2021), ‘Small and Simple, yet Sturdy: Conformationally Constrained Peptides with Remarkable Properties’, International Journal of Molecular Sciences, 22(4), Article 4.
- WangLet al (2022), ‘Therapeutic peptides: Current applications and future directions’, Signal Transduction and Targeted Therapy, 7(1), Article 1.
- FisherEet al (2019), ‘Peptide-Based Therapeutics for Oncology’, Pharmaceutical Medicine, 33(1), 9–20.
- IyengarSet al (2017), ‘The role of calcitonin generelated peptide in peripheral and central pain mechanisms including migraine’, Pain, 158(4), 543–559.
- Torres MDT et al (2019), ‘Peptide Design Principles for Antimicrobial Applications’, Journal of Molecular Biology, 431(18), 3547–3567.
- LiSet al (2023), ‘Therapeutic Peptides for Treatment of Lung Diseases: Infection, Fibrosis, and Cancer’, International Journal of Molecular Sciences, 24(10), 8642.
- GriecoPet al (2019), ‘Natural and synthetic peptides in the cardiovascular diseases: An update on diagnostic and therapeutic potentials,’ Archives of Biochemistry and Biophysics, 662, 15–32.
- ImaiKet al (2006), ‘Comparing antibody and smallmolecule therapies for cancer’, Nature Reviews, 6(9), 714–727.
- Lee AC et al (2019), ‘A Comprehensive Review on Current Advances in Peptide Drug Development and Design,’ International Journal of Molecular Sciences, 20(10), 2383.
- WangXet al (2021), ‘Rational Design of Peptide-Based Inhibitors Disrupting Protein-Protein Interactions’, Frontiers in Chemistry, 9.
- Wentink MQ et al (2016), ‘Targeted vaccination against the bevacizumab binding site on VEGF using 3D-structured peptides elicits efficient antitumor activity’, Proceedings of the National Academy of Sciences of the United States of America, 113(44), 12532–12537.
- Lau YH et al (2014), ‘Peptide stapling techniques based on different macrocyclisation chemistries’, Chemical Society Reviews, 44(1), 91–102.
- MoiolaMet al (2019), ‘Stapled Peptides—A Useful Improvement for Peptide-Based Drugs’, Molecules, 24(20), 3654.
- NuijensTet al (2019), ‘Natural Occurring and Engineered Enzymes for Peptide Ligation and Cyclization’, Frontiers in Chemistry, 7, 829.
- BechtlerCet al (2021), ‘Macrocyclization strategies for cyclic peptides and peptidomimetics’, RSC Medicinal Chemistry, 12(8), 1325–1351.
- TimmermanPet al (2009), ‘Functional Reconstruction of Structurally Complex Epitopes using CLIPS™ Technology’, The Open Vaccine Journal, 2(1), 56-67.
- Global Peptide Drug Market Peptide Drugs Sales Clinical Trials Insight 2028. (2022). BioSpace. Retrieved 23 January 2024, from https://www. biospace.com/article/global-peptide-drug-marketpeptide-drugs-sales-clinical-trials-insight-2028/
- ItohY (2006), ‘MT1-MMP:Akey regulator of cell migration in tissue’, IUBMB Life, 58(10), 589–596.
- CookNet al (2019), ’Pharmacokinetic (PK) assessment of BT1718:Aphase I/IIastudy of BT1718,afirst in class bicycle toxin conjugate (BTC), in patients (pts) with advanced solid tumours’, Annals of Oncology, 30, v174.

Peter Timmerman, head of peptide science at Biosynth, joined the group following the acquisition of Pepscan in 2022, where he had served as chief scientific officer since 2001. He drives advancements in protein mimicry for peptide drug discovery programmes. Timmerman is the inventor of CLIPS technology and previously held a chair as a Professor at the University of Amsterdam, the Netherlands. He obtained his Chemistry degree from Vrije Universiteit (Amsterdam, the Netherlands) and earned his PhD cum laude from the University of Twente, the Netherlands.