Product News – Winter Edition
IPT highlights some of the most recent exciting advancements in pharmaceutical manufacturing
Elevating 3D characterisation with enhanced performance and efficiencies – introducing the ZEISS VersaXRM 730 3D X-ray microscope

ZEISS launches its latest innovation in the field of X-ray microscopy: ZEISS VersaXRM 730. The new system offers researchers an unprecedented level of performance, choice and accessibility. The platform’s intuitive guidance and control system, the award-winning ZEN navx, takes accessibility to the next level, accommodating users of all skill levels. FAST Mode delivers one-minute tomographies, dramatically accelerating 3D imaging by using continuous sample motion data collection. The ZEN navx guidance and control system is a systematic approach of built-in guidance, automated workflows and intelligent system insights. Additionally, ZEN navx file transfer utility automatically transfers data from the microscope to other locations so that users have their information where they need it, when they need it. ZEISS VersaXRM 730 is also equipped with game-changing AI, unlocking entirely new application capabilities.
Nuclera’s eProtein Discovery system installed at Domainex to streamline protein production services
Nuclera and Domainex have announced that Domainex is the first contract research organisation to have installed the eProtein Discovery system and offer protein production services using the technology. Nuclera’s eProtein Discovery system is designed to speed up protein expression and purification optimisation in research labs, particularly those using AI including ML for protein design. Applying digital microfluidics alongside in situ protein detection assays and cell-free protein synthesis, eProtein Discovery enables rapid, scalable access to high-quality proteins, supporting both cell-free and cellbased expression methods. The system significantly reduces time and cost of these processes by automating construct screening, protein scale-up, and producing purified proteins for downstream functional testing in under 48 hours.
Asymchem’s fully automated peptide production lines set new benchmarks as large-scale capacity continues to grow

Asymchem has achieved fully automated manufacturing to further its large-scale peptide drug production. An initial hurdle – equipment scale-up – was addressed by Asymchem’s Chemical Engineering Department (CED) utilising advanced simulation techniques and experimental validation to finalise the solid-phase synthesiser design, and defining the stirring paddles’ structural characteristics. The implementation of an automated formulation system was an additional challenge which was overcome. Asymchem also developed a host computer system that seamlessly interfaces with the control system, facilitating unmanned production across the entire solid-phase synthesis process. Asymchem’s Center for Intelligent Manufacturing Technology (CIMT) and Center for Continuous Flow Chemistry Technology (CFCT) established communication links between necessary systems, eliminating information silos and ensuring coordinated control across the entire plant.
Ardena’s new state-of-the-art nanomedicine facility secures full good manufacturing practice approval for manufacturing

Ardena has announced the the full GMP approval of its expanded nanomedicine facility in Oss, the Netherlands. The approval follows a €20m investment in a state-of-the-art 45,000ft2 facility offering GMP-compliant Grade C and Grade D cleanrooms designed specifically for nanomedicine manufacturing. The fit-for-purpose cleanrooms, alongside additional GMP production spaces, dedicated laboratories for process development and analytical capabilities, and advanced warehouse facilities, reflect Ardena’s commitment to meeting stringent regulatory standards, including the latest updates to Annex 1 for the manufacture of sterile products. The Oss facility also includes purpose-built automated manufacturing flows, and fully integrated analytical capabilities for characterising complex nanomedicine formulations.
Strategic TaBlitz partnership with BIOGRUND

TaBlitz is excited to introduce its newest platform partner, BIOGRUND, a globally recognised leader in excipient technology. This exclusive partnership is set to bring significant value to TaBlitz’s ecosystem, enhancing its capabilities in tablet development. BIOGRUND’s specialisation in creating homogeneous mixes of excipients and carriers will introduce a wealth of knowledge and innovation to our platform. Its expertise in film coatings, sugar-coatings and tablet excipients provide its customers with access to high-quality formulation aids. Its ready-to-use excipient premixes will allow for quicker formulation adjustments, faster scale-up and reduced development times, enhancing the speed at which new products can reach the market. BIOGRUND’s commitment to sustainable practices and clean label formulations resonates with customers, offering options for formulations that avoid controversial ingredients like titanium dioxide, aligning with global health trends and consumer preferences.
Refeyn launches software platform to automate mass photometry analysis workflows
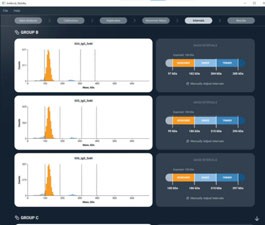
Refeyn has developed and launched a custom software platform providing automated analysis of large mass photometry data sets. Refeyn’s new StreamlineMP software suite offers specialist analysis modules, automating data processing through standardised workflows for key bioanalytical applications to deliver more consistent results in a shorter time. StreamlineMP is a complementary modular software platform to DiscoverMP , Refeyn’s data analysis software which accommodates a wide range of analytical options. DiscoverMP offers broad flexibility that is highly desirable for many users, yet for some, who want a standardised analysis workflow across a large number of data sets, it can prove time-consuming. Through its suite of application-specific module options, StreamlineMP supports these customers who need to apply repeated analysis parameters to large data sets for individual applications. Its use reduces analysis time and ensures the consistency of analytical parameters.