Manufacturing: protein-based immunotherapies
Protein-based immunotherapies – the journey from discovery to manufacturing
Protein-based immunotherapies, such as monoclonal antibodies and adoptive cell transfer therapies, are reshaping oncology treatments, but ensuring their safety and efficacy is crucial for their success
Chelsea B Pratt and Marwan A Alsarraj at Bio-Rad Laboratories
Immunotherapy strategies involving protein-based molecules such as monoclonal antibodies (mAbs) (eg, immune checkpoint blockade [ICBs] drugs) and adoptive cell transfer therapies (eg, CAR T-cell therapy) are reshaping cancer treatment. Unlike conventional treatments such as radiation and chemotherapy, immunotherapy functions by either augmenting the native immune system or introducing engineered components to fortify defences against cancer. However, achieving success in bringing a new therapeutic to market hinges upon the ability to meticulously monitor the impact of an immunotherapeutic across cells, genes and proteins, taking into consideration complex biological pathways and diverse cell types involved. Assessing a lead candidate’s clinical potential involves navigating through the unique challenges associated with each stage of development, from inception to completion.
Key considerations for preclinical development
Identifying promising therapeutic candidates mainly involves employing a phenotype-based or target-based approach. Phenotypic screening takes a target-agnostic approach to identify novel candidates putatively associated with a therapeutic effect. On the other hand, a target-based approach utilises high-throughput screening for the discovery of therapeutic candidates for specific targets associated with disease. A notable example of this approach includes the use of phage display technology, a powerful in vitro drug discovery platform, instrumental for the identification of potent fully human therapeutic mAbs. Recent advances in phage display technology led to the development of sophisticated antibody libraries that surpass the normal human immune repertoire, offering the potential to expedite the identification of superior therapeutic antibodies with exceptional affinity.
During the lead discovery stage, purified potential drug candidates undergo comprehensive evaluation across a range of properties, including efficacy, specificity, selectivity, pharmacokinetics (PK), pharmacodynamics (PD) and immunogenicity. Once a lead candidate has been selected, further characterisation testing is conducted and additional lead optimisation techniques, such as protein engineering, can be employed to potentially enhance a therapeutic’s efficacy and safety.
Lead candidates must ideally demonstrate the ability to effectively engage the target and modulate its activity in vivo and in vitro while maintaining a favourable safety profile. Ligand-binding assays (LBAs) are commonly employed to assess drug efficacy, potential toxicity and optimal dosing regimens. Additionally, anti-drug antibody (ADA) assays are conducted to detect antibodies produced by the immune system in response to the drug that may negatively impact drugs’ efficacy. These assays, designed in accordance with regulatory requirements (eg, ICH M10 Bioanalytical method validation and study sample analysis), establish safety parameters guiding the therapeutic throughout further development.
The quality of detection reagents used in LBAs significantly impacts the success of this stage of development. Fully human anti-idiotypic (anti-ID) antibodies derived by phage display are ideal for these assays because they provide greater specificity than traditional antibodies. Moreover, as these antibodies are produced recombinantly using fully in vitro technology providing sequence identity, they ensure greater batch-to-batch consistency and facilitate the development of robust assays suitable for the entire developmental process, eliminating the need for use of animal-derived components or laboratory animals.
Assessing drug safety and efficacy via immune cell analysis
Assessing drug safety and efficacy through immune cell analysis is integral during the early stages of immunotherapy development. Comprehensive cell-based assays are essential for thoroughly understanding a drug’s effects across various pathways and cell types. Utilising analytical tools capable of automation and simultaneous data collection across multiple parameters support complex and informative multiplexed analysis. High-throughput cell phenotyping using flow cytometry has emerged as a crucial method for detecting and quantifying diverse immune cell populations within heterogeneous samples. This technology offers simultaneous detection of an expanding array of cell markers, facilitated by flow cytometers equipped with multiple lasers and detectors.
Moreover, instruments designed for automation further optimise high-throughput capabilities, streamlining operations and improving process efficiency. This is particularly useful for preclinical applications requiring compliance with regulatory standards (eg, 21 CFR Part 11).
With a broad range of research and clinical applications, flow cytometry plays a pivotal role in assessing the safety and efficacy of immunotherapies, but also understanding the immune system’s role in disease progression and monitoring patient responses to treatment. However, working with complex, multiparameter panels requires careful consideration of instrument capabilities, fluorophore properties, sample availability and antibody functionality. Careful panel design is essential in immunophenotyping to mitigate complexities introduced by additional fluorophores, which could compromise data accuracy. As such, selecting suitable antibodies and reagents is critical for successful high-throughput screening.
Moreover, leveraging online tools has also been underscored as a valuable resource to simplify the intricate process of panel design. While the process may involve multiple design and optimisation steps, adhering to best-practice guidelines and selecting appropriate controls can make the process more efficient and cost-effective ultimately leading to reproducible and reliable experimental outcomes.
Multiplexed assays for cytokine analysis
Multiplex immunoassays such as the bead-based cytometry method offers simultaneous data on multiple immune response targets, providing faster results and requiring smaller sample volumes than single target assays such as ELISA. This technology is invaluable when working with limited patient samples, allowing for the simultaneous testing of multiple analytes and enhancing sensitivity to detect secreted cytokines, chemokines and other proteins from small quantities of serum samples. The precise and rapid acquisition of patient data afforded by multiplex immunoassays can mitigate serious complications and provide evidence-based insights for developing protein-based immunotherapies. Specifically, understanding the role of signalling proteins like cytokines or chemokines in the immune system and how they respond to immunotherapeutic interventions is crucial for developing optimal treatments. Maximising assay throughput and studying the relationship between multiple proteins using small sample volumes are key objectives for researchers exploring complex immune networks.
Western blotting analysis of protein expression
Western blotting is another versatile tool aiding in the development, validation and optimisation of protein-based immunotherapies. Researchers can use it to assess protein expression, modifications, interactions and even quality. For example, western blotting can differentiate between isoforms based on their molecular weights, as well as detect phosphorylated proteins. Stain-free western blotting offers a streamlined workflow for assessing protein expression more rapidly and accurately than conventional western blotting methods. This advanced technique enhances efficiency in protein analysis, facilitates the elucidation of molecular mechanisms underlying target therapeutic pathways and aids in uncovering new targets for next-generations therapies.
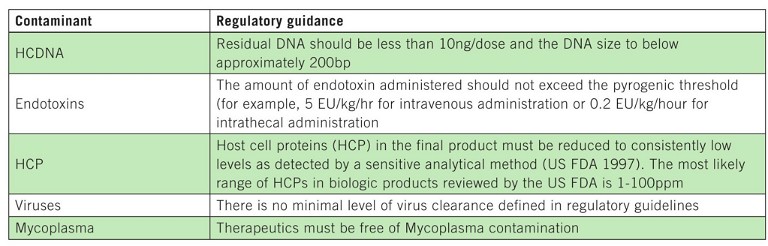
Table 1: Impurities that may be present in bioprocessing workflows and limits defined by regulatory authorities
Monitoring response to immunotherapy and biomarker discovery
Understanding and predicting how a tumour will respond to a therapeutic is critical to drug development. Genomic analysis in preclinical and clinical studies allows for the identification of genetic markers associated with treatment, either positive or negative, uncovering potential biomarkers indicating treatment response or disease progression, providing essential insights into immunotherapy mechanisms. By monitoring responses, clinicians can identify patients at higher risk and adjust treatment strategies. Clinicians can also adjust dosages, combine therapies or switch treatments based on genomic data to achieve the best possible outcomes.
Various techniques can be employed to genetically monitor responses:
• Quantitative PCR (qPCR), an industry standard, allows simultaneous measurement of multiple genes from various samples, including liquid biopsy, tissue or cell-based materials. This method is indispensable for comprehensive genomic analysis, relying on fluorescent reporter molecules to quantify DNA product accumulation in real time
• Digital PCR (dPCR) enhances sensitivity and offers absolute quantification, crucial for detecting rare mutations or low-abundance biomarkers. By partitioning samples into nano-reactions, dPCR generates thousands of data points from a single sample, facilitating statistical analysis without standard curves and increases the ability to find rare targets amongst a complex background
• Next-generation sequencing (NGS) stands out as an ideal approach for screening purposes because, unlike PCR-based techniques, it does not require prior knowledge of gene sequences. NGS technologies, such as whole genome sequencing (WGS) and RNA sequencing (RNA-seq), enable comprehensive profiling of genetic and transcriptomic landscapes, allowing researchers to identify potential biomarkers that may influence therapeutic outcomes.
By leveraging advanced genomic technologies like NGS alongside targeted molecular assays such as qPCR and dPCR, researchers can decipher intricate genomic responses to immunotherapy and identify biomarkers to inform and optimise treatment strategies. These assays can provide comprehensive transcript coverage and can aid in the development and optimisation of targeted treatments.
Ensuring product safety during manufacturing and QC
Demonstrating the purity and potency of a drug throughout its development is crucial for ensuring its safety and effectiveness. Common impurities in protein-based immunotherapies include host cell proteins (HCPs), DNA from expression systems, viruses, Mycoplasma and endotoxins. Additionally, impurities like leached Protein A from chromatography columns, extractables, buffers and detergents may be introduced during production, underscoring the need for a systematic purification approach integrating various strategies.
The resin chosen for each purification step must be compatible with specific challenges associated with that stage of the process. Downstream purification steps following protein capture depend on the molecule’s biophysical properties as well as any process-related impurities that must be removed. For example, residual host cell DNA (hcDNA) may co-copurify during the initial capture step requiring an intermediate anion exchange resin or membrane to remove this impurity from the target biomolecule. If hcDNA is carried over into the final product, it increases the risk of immunogenicity.
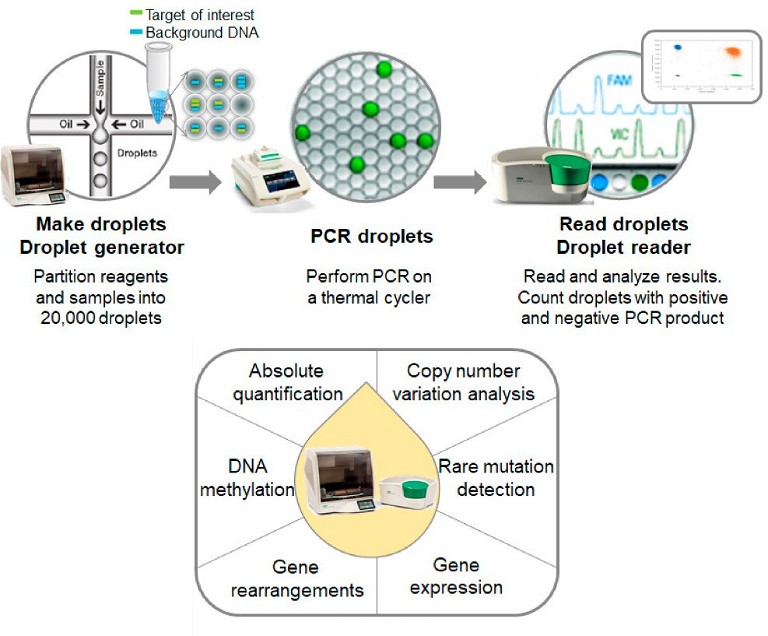
Figure 1: Overview of nucleic acid screening process using ddPCR. Adopted from Huerta et al 2021, under a CC BY 4.0.
Consequently, regulatory agencies, like the US Food and Drug Administration (FDA), mandate therapeutic products to contain no more than 10ng of hcDNA per dose, necessitating accurate quantification to ensure product safety (Table 1). Thus, chromatography is an integral component in the development of a robust drug production process throughout the drug development process, from discovery to manufacturing.
When it comes to large-scale immunotherapy production pipelines, it is also important to take into consideration elements that could increase downstream process efficiency. For example, resins tailored for a wide range of protein-based therapeutics enable targeted purification to achieve desired purity, efficacy and stability levels. Moreover, these resins often offer economic benefits by optimising process efficiency and are available in diverse formats suitable for scalable commercial production. In addition, adoption of automated chromatography systems leveraging multidimensional (multi-D) chromatography offering consistent and reproducible purification workflows, may lead to improved costand time-efficiency in screening and research settings. Such streamlined processes could ultimately accelerate introduction of products into the market.
Traditional nucleic acid detection methods like qPCR for hcDNA monitoring have drawbacks such as amplification bias, non-specific signal and lack of reproducibility, necessitating sample extraction and calibration standards. In contrast, droplet-based dPCR counts nucleic acid molecules encapsulated in individual droplets, providing absolute quantification without relying on standard curves.
This approach offers high precision, accuracy and sensitivity, making it ideal for measuring trace amounts of target DNA. It can also be employed throughout development and manufacturing to detect contaminants like adventitious agents and Mycoplasma in addition to hcDNA, all of which may compromise product safety.
Forward planning for future therapies
Ensuring the safety and efficacy of immunotherapies is critical for their success, with bioanalytical, quality control and purification processes playing pivotal roles from earlystage research to final production. Investing in reliable, established tools with proven track records mitigates risks of unexpected regulatory hurdles and ensures the generation of high-quality data.
This approach minimises safety risks, product recalls and costly delays in regulatory approval and fosters confidence in the therapeutic’s performance. By adhering to stringent guidelines and leveraging advanced tools and methods, companies can uphold the highest standards of safety and efficacy for therapeutic antibodies, ultimately benefiting patients and advancing the field of immunotherapy.

Marwan Alsarraj is the translational research segment manager at Bio-Rad. He has been at the forefront of developing, marketing and commercialising technologies for the past 18 years in the life sciences research industry. Marwan obtained his MS in biology at the University of Texas, El Paso, US.

Dr Chelsea Pratt is the biopharma market development manager at Bio-Rad Laboratories. In this role, she works with leaders in the biotech and pharmaceutical industries to accelerate drug discovery and development in new therapeutic areas. She is a recognised expert in chromatography and protein purification, and serves as a regular panellist at conferences, seminars and webinars. Dr Pratt holds a PhD in Biological Chemistry from the University of Texas Southwestern Medical Center, US.