Lab Design: Microbiological Monitoring
Rapid microbiological monitoring: transforming environmental monitoring in pharmaceutical manufacturing
Ensuring a manufacturing environment stays clear of bacteria and fungi is of utmost importance, especially when it comes to pharmaceuticals. How can this highly specialised monitoring be achieved in a fast, cost-effective manner?
Yoggya De Silva at Cherwell Laboratories
In the pharmaceutical industry, maintaining a sterile manufacturing environment is not only a regulatory obligation, but a crucial component of product quality and patient safety. Contaminants such as bacteria and fungi can compromise the sterility of medicinal products, leading to serious health risks. As pharmaceutical manufacturers strive to meet stringent regulatory standards, the implementation of effective environmental monitoring (EM) strategies is paramount.
Among the innovative tools helping to shape the future of environmental monitoring, rapid microbiological monitoring (RMM) methods such as biofluorescent particle (BFP) counters – which can deliver results in real time and support continuous monitoring – offer significant advantages over traditional monitoring methods.
EM in pharmaceutical manufacturing
EM is an essential practice for pharmaceutical manufacturers for several reasons. Firstly, it ensures the sterility of products by maintaining a clean manufacturing environment. The presence of contaminants can lead to product quality issues and pose risks to patient safety.
Regulatory bodies, such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have established rigorous guidelines for EM, making compliance vital if manufacturers are to avoid penalties and maintain their respective market positions.
Beyond regulatory compliance, continuous EM provides valuable data that can be analysed to identify trends and address potential issues before they escalate. This proactive approach helps maintain high quality standards throughout the manufacturing process.
Additionally, EM plays a critical role in risk management by identifying and controlling potential contamination sources. By monitoring air, surfaces and personnel, manufacturers can implement corrective actions to mitigate risks effectively.
Finally, the data generated through EM is crucial for batch release decisions. If contamination is detected, affected batches may need to be rejected to safeguard product integrity and ensure patient safety.3
RMM and real-time BFP counters
Traditional EM methods, such as culture-based techniques, have been a reliable standard for decades. They are known, trusted and understood in EM. However, these methods have shortcomings. In particular, there are often delays due to the necessary microbial incubation period, with results taking several days to process.
By contrast, RMM including BFP counters that can detect both inert and microbial particles in real time, by using laser-induced fluorescence to differentiate viable particles emitting fluorescence (Figure 1), offer distinct advantages.
One of the primary benefits of RMM and BFP counters, such as the bioaerosol monitoring system (BAMS) (Figure 2), is the speed of detection and delivery of contamination alerts to users. By providing real-time results, these advanced technologies enable manufacturers to take immediate corrective actions and reduce the risk of contamination spread.4
Additionally, such technologies exhibit heightened sensitivity and specificity in detecting viable microorganisms, as they can differentiate between biological particles and inert matter, yielding more accurate data.5
Enhanced efficiency through continuous monitoring and real-time data
Continuous and non-disruptive monitoring is another key advantage of these rapid technologies. This is because traditional methods typically rely on periodic sampling, which can miss transient contamination events. Conversely, RMM and BFP systems can be integrated with manufacturing control systems, facilitating automated data collection, analysis and reporting, which enhances data integrity and supports regulatory compliance.6 Moreover, the real-time data provided by RMM and BFP counters can significantly reduce the downtime associated with traditional testing.
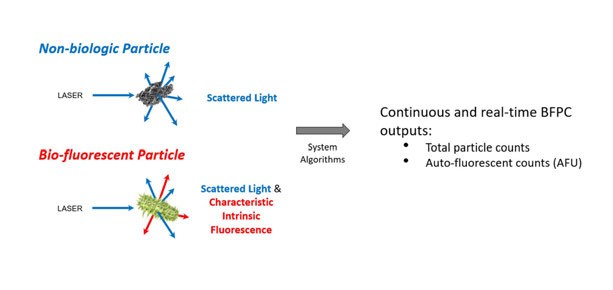
Figure 1: The BFP counter method uses light-scattering to detect non-biologic and biofluorescent particles, providing total particle counts and autofluorescent counts (AFU)
With immediate feedback, manufacturers can act quickly to address contamination issues, leading to increased productivity and cost savings. Importantly, BFP counters eliminate the need for reagents and significant hands-on time, making them a more efficient alternative to traditional growth-based methods of EM.7
Validation of new RMM technology in EM programmes
While the benefits of RMM and BFP technologies are clear, the introduction of new monitoring systems into existing EM programmes necessitates a thorough validation process.
Figure 2: Biofluorescent particle counters, such as BAMS, can enable RMM to provide near real-time results to support immediate corrective actions, minimising the risk of contamination spreading
Manufacturers should adopt a systematic approach that begins with a comprehensive risk assessment, in order to understand the impact of the new technology on their EM programme. Identifying potential challenges is crucial to ensure a smooth transition.6
A detailed validation plan is essential, outlining objectives, methodologies, acceptance criteria and responsibilities, while ensuring alignment with internal and external regulatory requirements. Validation involves three main phases: installation qualification (IQ), operational qualification (OQ) and performance qualification (PQ):5,6
• IQ verifies that the system is installed according to manufacturer specifications
• OQ assesses whether the system operates as intended within the defined parameters
• PQ determines whether the system meets user requirements for EM.
The PQ step often includes comparative studies between new RMM technology and traditional methods to demonstrate equivalence or superiority in detecting and quantifying microorganisms. Robust testing under various conditions ensures consistent and reliable technology performance.6,7 Training personnel on new technology is vital for its effective operation, maintenance and data interpretation.
Manufacturers of RMM technologies typically provide guidelines for validating their specific products across various applications. Continuous performance review post-implementation is crucial for maintaining the effectiveness of the new technology.
Documentation of the validation process, including test results, deviations and corrective actions, is vital for regulatory compliance. The process should culminate in a validation report summarising findings and conclusions, ensuring transparency and accountability.5,6
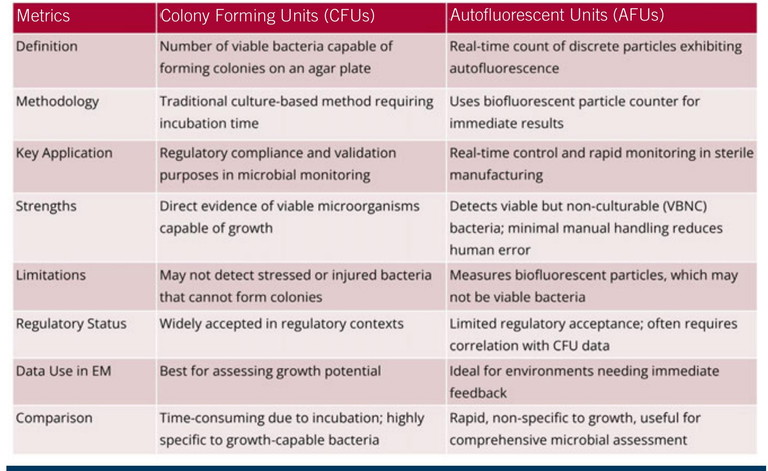
Table 1: A comparison of CFUs and AFUs
Comparing CFUs and AFUs
Colony forming units (CFUs) and autofluorescent units (AFUs) are two metrics used to quantify bacterial concentration in sterile manufacturing environments, but they represent fundamentally different measurement approaches.
CFUs refer to the number of viable bacteria capable of forming colonies on an agar plate – a traditional method of EM that requires incubation time. This approach has been widely accepted for regulatory compliance and validation purposes, as it directly evidences viable microorganisms capable of growth.8
By contrast, AFUs provide a real-time count of discrete particles exhibiting autofluorescence, indicating biological activity. This measurement, typically obtained using a BFP counter, delivers rapid results.
While both CFUs and AFUs serve as estimations, the underlying methodologies differ significantly, making them non-equivalent.9 Table 1 provides a comparison of the two metrics.
CFUs may fail to detect stressed or injured bacteria that are unable to form colonies under given culture conditions. Conversely, AFUs can identify viable but non-culturable (VBNC) bacteria and other BFPs, offering a more comprehensive assessment of microbial presence.
The automated nature of AFU measurement reduces manual handling and potential human error associated with CFU counting.8,10
While CFUs provide a direct measurement of growth potential, AFUs are valuable for rapid monitoring and real-time control, particularly in environments where immediate feedback is essential. However, regulatory acceptance of AFU data may require correlation with CFU data, posing challenges due to the methodological differences.9
Mitigating risks in sterility testing
Sterility testing in pharmaceutical manufacturing involves various risks that can compromise result reliability. Contamination from the environment, personnel or equipment can lead to false-positive results. Implementing stringent aseptic techniques, utilising cleanroom environments and conducting regular personnel training on contamination control practices, can effectively mitigate these risks.9
False negatives – instances where tests fail to detect viable microorganisms due to inadequate sampling or the presence of VBNC bacteria – can be addressed by integrating complementary testing methods, such as BFP counters, into the testing regime.6
It is also essential to identify potential false positives associated with RMM and BFP counters, such as ambient particles or machine sensitivity challenges. Certain laboratory equipment can generate fluorescent signals, potentially skewing results. So, comprehensive validation processes should include testing of materials present in the sampling area to establish baselines for false-positive counts.10
Human error in sample collection, handling or interpretation poses another significant risk. Standardising procedures, automating processes and providing thorough training for personnel can help mitigate this risk. Furthermore, equipment failure, which can lead to inaccurate results or testing delays, underscores the importance of regular maintenance and calibration.9
By adopting stringent procedures, ongoing training and leveraging advanced technologies, pharmaceutical manufacturers can enhance the reliability and accuracy of their sterility testing processes. The integration of BFP counters and other RMM technologies into EM programmes will lead to improved product quality, regulatory compliance and, ultimately, patient safety.
Real time monitoring in EM moving forwards
As the pharmaceutical industry continues to evolve, the importance of robust environmental monitoring systems cannot be overstated. Real-time BFP counters represent significant advancements in the field, offering rapid, accurate and non-disruptive monitoring solutions that can transform the way manufacturers ensure sterility and compliance. The shift from traditional microbial monitoring methods to real-time solutions reflects a broader trend towards technological innovation in pharmaceuticals. Embracing these advancements will enhance regulatory compliance and also drive operational efficiencies that can translate into substantial cost savings.
By providing immediate feedback on contamination levels, RMM systems such as BFP counters can mitigate risks before they escalate into significant issues. This proactive approach not only safeguards product integrity but also bolsters consumer trust in pharmaceutical products.
Integration of real-time monitoring systems supports a culture of continuous improvement within manufacturing processes. As companies adopt these technologies, they can gather comprehensive data that informs decision-making and operational strategies. This data-centric approach empowers manufacturers to refine their processes continually, ensuring that they not only meet but exceed industry standards.
In addition to improving compliance and operational efficiency, these technologies contribute to enhanced patient safety. The pharmaceutical industry bears a significant responsibility for delivering safe and effective products to the market. By utilising advanced monitoring systems, manufacturers can better protect public health and prevent contamination-related incidents that could have far-reaching consequences.
As the industry moves forward, collaboration between manufacturers, technology providers and regulatory bodies will be essential to fully harness the potential of real-time environmental monitoring systems. Through a collaborative approach, stakeholders can ensure that RMM innovations are implemented effectively and also aligned with regulatory expectations – ultimately paving the way for a more secure and reliable pharmaceutical landscape.
Conclusion
Investing in RMM technologies such as real-time BFP counters is not just a technical upgrade; it is a strategic imperative that aligns with the industry’s commitment to quality, safety and innovation. The future of pharmaceutical manufacturing lies in the ability to adapt to changing demands and leverage technological advancements that enhance both process efficiency and product quality. As manufacturers navigate this dynamic landscape, the importance of adopting and validating these systems will be key to achieving long-term success and sustainability in the highly competitive pharmaceutical industry.
References
- Visit: fda.gov/media/71026/download
- Visit: ema.europa.eu/en/documents/scientific-guideline/guideline-sterilisation-medicinal-product-activesubstance-excipient-and-primary-container_en.pdf
- MoldenhauerJ (2011), ‘Environmental Monitoring:A Comprehensive Handbook’, DHI Publishing
- Visit: pubmed.ncbi.nlm.nih.gov/30361285/
- Visit: jddtonline.info/index.php/jddt/article/view/1858
- Visit: pmeasuring.com/wp-content/uploads/2019/03/224_usp-1223-validation-ofalternative-micro-metho.pdf
- Visit: pubmed.ncbi.nlm.nih.gov/31843986/
- Visit: https://pubmed.ncbi.nlm.nih.gov/35840344/
- Visit: https://pubmed.ncbi.nlm.nih.gov/34531294/
- Visit: https://pubmed.ncbi.nlm.nih.gov/37451837/

Yoggya De Silva, microbiology product specialist at Cherwell Laboratories, holds extensive experience in product management and microbiological solutions across sterile medical products, active pharmaceutical ingredients and biologics manufacturing. With a biochemistry background and an MSc in immunology, she has worked with companies, including Lonza, Nova Biomedical and SaniSure, to build her strong industry knowledge.