Manufacturing: protein synthesis in development
The role of protein synthesis in drug discovery and development
Protein synthesis is the process by which cells build proteins, fundamental molecules necessary for virtually all cellular functions, but how can this be used within the discovery and manufacturing of new drugs and how can it be automated?
Michael Chen at Nuclera
IPT: What is protein synthesis and how is this currently used in drug discovery and development?
Michael Chen (MC): Protein synthesis is the process by which cells build proteins, fundamental molecules necessary for virtually all cellular functions. The process involves two main stages: transcription, where DNA is transcribed into messenger RNA (mRNA), and translation, where mRNA is decoded by ribosomes to synthesise proteins. Proteins perform a variety of roles, including acting as enzymes, structural components and signalling molecules, making them crucial targets and tools in drug discovery and development.
In drug discovery, target proteins and therapeutic proteins are needed at microgram to gram scale at high purities. Protein synthesis is used to express proteins in cells and purify these proteins to high purity to meet the needs for drug discovery. Target proteins are those that are investigated as potential points of intervention for new drugs. For example, the commonly used, over-the-counter painkiller ibuprofen acts by binding and inhibiting to target proteins called cyclooxygenase enzymes within the human body. By synthesising target proteins in the lab, researchers can study their structure, function and interactions with potential drug candidates, allowing for the identification of compounds that modulate the protein’s activity.
Therapeutic proteins, on the other hand, are designed to treat diseases directly, including monoclonal antibodies, insulin and growth factors, among others. The ability to synthesise these proteins reliably and at scale is essential for their development and commercialisation. Advances in recombinant DNA technology have also allowed for the production of proteins that are not naturally occurring or are difficult to extract from natural sources – further expanding the range of therapeutic proteins available. Protein synthesis is integral to the development of high-throughput screening assays, where large libraries of compounds are tested for their ability to interact with or inhibit the function of target proteins. This accelerates the identification of promising drug candidates, streamlining the drug discovery process.
IPT: Why is protein synthesis vital to drug discovery?
MC: Protein synthesis is vital to drug discovery for several reasons. Many diseases are caused by the malfunction or dysregulation of proteins, therefore synthesising proteins is crucial in helping us understand disease mechanisms. By synthesising and studying proteins that are malfunctioning or dysregulated, researchers can gain insights into the underlying mechanisms of diseases, which is important for identifying new therapeutic targets. Laboratory expressed and purified proteins allow for the validation of potential drug targets. Researchers can manipulate these proteins in vitro to understand their role in disease and determine whether modulating their activity with a drug could have therapeutic benefits. Protein synthesis also supports high-throughput screening. By synthesising target proteins and developing assays around them, researchers can screen thousands to millions of compounds to identify potential drug candidates.
Nearly all drug discovery programmes today require detailed structural information about target proteins to create and optimise new drugs. The ability to produce large quantities of proteins enables these structural studies through laboratory techniques such as macromolecular X-ray crystallography and cryo-electron microscopy. Access to structural information and other characteristics about a target protein enables structure-based drug design, where drugs are designed to fit precisely into the active site of a protein, enhancing their efficacy and reducing off-target effects.
IPT: In what ways can this process be automated?
MC: Automation of protein synthesis can be achieved using robotic systems, advanced software and integrated platforms to streamline and scale the expression, purification and analysis of proteins. Automation can be applied across key areas including:
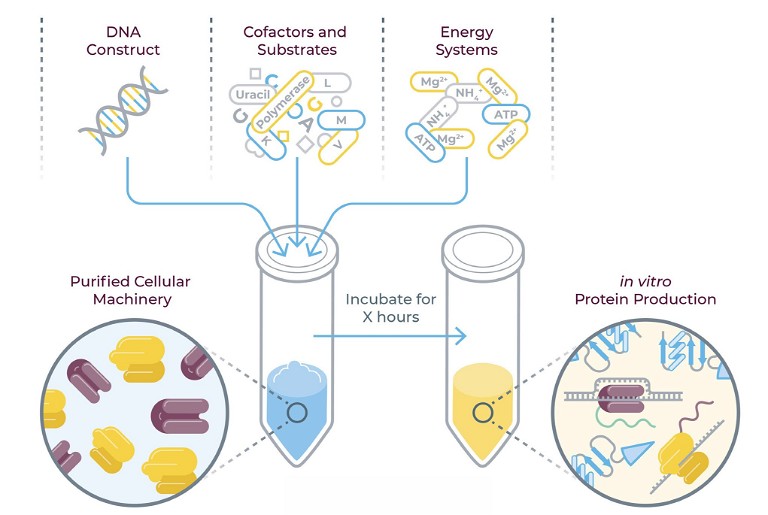
1. Gene synthesis and cloning: Automated platforms can rapidly synthesise and clone genes encoding target proteins. These systems use high-throughput techniques to construct DNA sequences and insert them into expression vectors, reducing the time and labour required compared to manual cloning
2. Protein expression: Automating protein expression involves the use of robotic systems that can handle large numbers of samples whilst optimising conditions (eg, temperature and media composition) to maximise protein yield. These systems can operate in parallel, significantly speeding up the production process
3. Protein purification: Robotic platforms can handle multiple purification steps, ensuring high purity and consistency. Automation also allows for the rapid screening of different purification conditions to optimise yield and quality
4. High-throughput screening: Automation is crucial in high-throughput screening, where large libraries of compounds are tested for activity against target proteins. Robotic systems can dispense reagents, incubate samples and read assay results quickly and accurately, enabling the screening of thousands to millions of compounds in a short time
5. Data integration and analysis: Advanced software tools integrate data from various stages of protein synthesis and analysis, providing a comprehensive overview of the process. Machine learning algorithms can be used to predict optimal conditions for protein expression and purification, further enhancing efficiency.
IPT: What are the benefits of increased automation in protein synthesis, specifically in terms of drug discovery?
MC: ‘Drug discovery begins with protein’. Without the target protein in hand, drug discovery cannot begin. Automation significantly reduces the time required for the traditionally laborious and people-intensive protein expression, purification and analysis process. High-throughput automated systems can process multiple samples simultaneously, allowing researchers to complete projects in weeks that would otherwise take months or even years. This speed and efficiency is coupled with consistency and reproducibility – automated systems minimise human error and variation, which is crucial in drug discovery, where reproducibility is essential for validating findings and moving potential drug candidates through the development pipeline. Furthermore, despite extensive preclinical testing, the vast majority of drugs that enter clinical trials ultimately fail to win approval. The reasons for failure are complex but can often be attributed to the wrong target being chosen.
Scaling the protein synthesis process is an ever-growing need as increasingly vast libraries of compounds are screened, which require large-scale production of target proteins. Rapid screening of thousands of compounds against multiple targets increases the likelihood of identifying effective drug candidates and reducing the time to market for new therapies. Automation enables the scalability of protein synthesis but also is cost-effective in the long run. Whilst the initial investment in automated systems can be high, the long term-cost savings are substantial due to reduced labour costs and decreased amounts of reagents and consumables needed.
Automated platforms often include sophisticated data integration and analysis tools that can process large data sets generated during protein synthesis and screening, providing valuable insights into protein behaviour, interactions and potential drug efficacy. This integrated approach facilitates more informed decision-making and accelerates the identification of promising drug candidates. Ultimately, by freeing researchers from routine tasks, automation allows them to focus on more complex and creative aspects of the drug discovery process. This can lead to innovative approaches and new discoveries, as scientists have more time and resources to dedicate to hypothesis-driven research and experimental design.
IPT: What challenges are facing innovations in this area?
MC: Protein synthesis is a complex and laborious process – most proteins are not easily expressed and purified. These challenges are complicated by the fact that protein expression and purification are difficult and expensive to automate. Membrane proteins, large multi-domain proteins and proteins requiring specific post-translational modifications can be challenging to produce in sufficient quantities and with correct folding and functionality.
Integrating different automated systems and ensuring their interoperability can also be complex due to different platforms using distinct protocols, formats and software. This integration is crucial, which would require the development of standardised interfaces and protocols. Added to that is the need for the development of robust protocols that account for biological variability that can impact protein yield and quality. Integration could be costly but so currently is the initial cost of setting up automated systems, which can be prohibitive. Ensuring that advancements in automation are accessible and affordable to a broad range of researchers is crucial for widespread adoption.
While automation generates a huge amount of data, the processes required for efficient storage, management and analysis can still be difficult to put in place. Developing robust data management systems and ensuring data quality and integrity are ongoing challenges. Technical expertise also poses a challenge as operating and maintaining automated systems require specialised skills. There is a need for ongoing training and development to ensure that researchers can effectively use these advanced technologies. As automation advances, there are regulatory and ethical considerations to address. Ensuring that automated processes comply with regulatory standards and ethical guidelines is critical for the development of safe and effective therapeutics.
IPT: How do you see this field developing in the next five years?
MC: Driven by ongoing technological innovations and increasing integration of automation and AI, the field of protein synthesis is poised for significant advancement over the coming years. The use of AI and machine learning will become more prevalent, optimising every stage of protein synthesis from gene design to expression and purification. AI will continue to predict protein folding, stability and interactions, leading to more efficient and targeted drug discovery processes.
Advances in high-throughput technologies will further accelerate the screening and analysis of proteins. As automated platforms become more sophisticated, capable of handling even larger libraries of compounds and more complex assays, the speed and accuracy of drug discovery will also increase. Efforts to standardise protocols and improve the interoperability of automated systems will gain momentum, which will facilitate integration across different platforms and perhaps even laboratories. Taken together, these will result in improved collaboration and data sharing amongst researchers and scientists. As technologies become more widely adopted, the cost of automation is expected to decrease, which will make advanced automated protein synthesis accessible to a broader range of research institutions, including smaller labs and startups. In addition, the applications of automated protein synthesis will expand beyond traditional drug discovery and into new therapeutic modalities, such as gene therapies, personalised medicine and novel biologics, as they benefit from these advancements and as costs reduce. Regulatory frameworks will also evolve to keep pace with technological innovations. Clear guidelines and standards for the use of automated systems in drug development should be established, ensuring that new therapeutics are developed safely and effectively.
The next five years, and beyond, will see significant advancements in the automation of protein synthesis, driven by technological innovations and a focus on integration, cost reduction and expanded applications. These developments will enhance the efficiency and effectiveness of drug discovery and development, leading to new and innovative therapies for a wide range of diseases.

Michael C Chen is CEO of Nuclera, and is a leading expert in protein biology with over 15 years of experience in the field. Michael holds a PhD in Molecular Biology from Cambridge University, UK, and has contributed to numerous groundbreaking research projects in protein synthesis and drug discovery. Currently, Michael is dedicated to advancing automated solutions for synthetic biology applications, driving innovation and efficiency in the development of new therapeutics.