Manufacturing: Tabletting
SPOT LIGHT
Excipient-Binder Scale for Direct Compression and Dry Granulation Tabletting
For different binder-diluent ratios on the tablet mechanical strength, a proposed scale could serve as a ‘guiding torch’ for various formulators while designing preclinical or early phase formulations
By Devang Patel, Kerry Cruz, Robert Sedlock and Rahul Haware at Natoli Scientific
A direct compression and dry granulation are the first and second choice of tablet manufacturing due to their simplicity as compared to the cumbersome wet granulation process.1 A direct compression has a limited number of inter-processing steps. It can therefore pose less challenges for continuous tablet manufacturing as per the ICHQ8 guidelines.2 One of the downsides of direct compression is its inability to process bulkier and poor-flowing powders. This can be handled with a second-choice dry granulation process.1 A dry granulation is also a ‘method-of-choice’ for various high-load bulkier drug formulations, specifically small molecule oncology new chemical entities (NCEs). Such NCEs belongs to BCS Class-II to Class-IV leading to their high dose. As per our experience, oncology tablet formulation could have API from 30% to 80%; this implies the 30-80% dominance of API on the final tablet formulation properties with limited room for the quantity of excipients. This warrants a smart selection of multipurpose excipients while developing the formulations to avoid phase 2 to late development tablet manufacturing issues such as capping, lamination, picking and sticking (CLPS).
CLPS issues are difficult to capture in the early development stage, becoming more prominent in the late-stage development phase thanks to large batch sizes, as compared to early phases. Such late-development issues become very challenging because of the boxed formulation. These troubled formulations are difficult to change owing to their involved time and money expenditure. Certainly, it is important to understand vital components of formulations, such as multipurpose excipients, even in the preclinical stage of formulation development. Tablets formulations are composed of two or more excipients serving a purpose of binder and diluent, providing the desired strength to maintain the tablet integrity. Common binder-diluents used in the formulations are microcrystalline cellulose, lactose, dicalcium phosphate dihydrate (Emcompress®) and mannitol. Formulation scientists employ different ratios of these materials to achieve a desired mechanical strength of the tablet for a robust tabletting. These binders are predominantly plastic and brittle in nature. Hence, it is important to evaluate the impact of a diluent-binder replacement on the compact strength of a directly compressible tablet. This beforehand knowledge could be extrapolated to the dry granulation process if necessary.
The aim of the present investigation was to evaluate the impact of different binder-diluent ratios on the tablet mechanical strength of resulting tablets. Finally, a binder-diluent classification scale was proposed. This scale could serve as a ‘guiding torch’ for various formulators while designing their direct compression or dry granulation formulations.
Materials and Methods
Different predominantly deforming various common binder-diluents such as microcrystalline cellulose (plastic), α-lactose monohydrate (low fragmenting), Emcompress® (high fragmenting) and mannitol (brittle) were chosen.3,4 These materials were sieved from Sieve #40 and then blended in 25:75, 50:50 and 75:50 ratios in a lab-scale V-shape blender. A single component and bi-component diluent-binder 10mm flat-faced tablets of these materials were manufactured by compressing powder at 100 MPa, 200 MPa and 300 MPa using a Natoli RD-10A single station press.
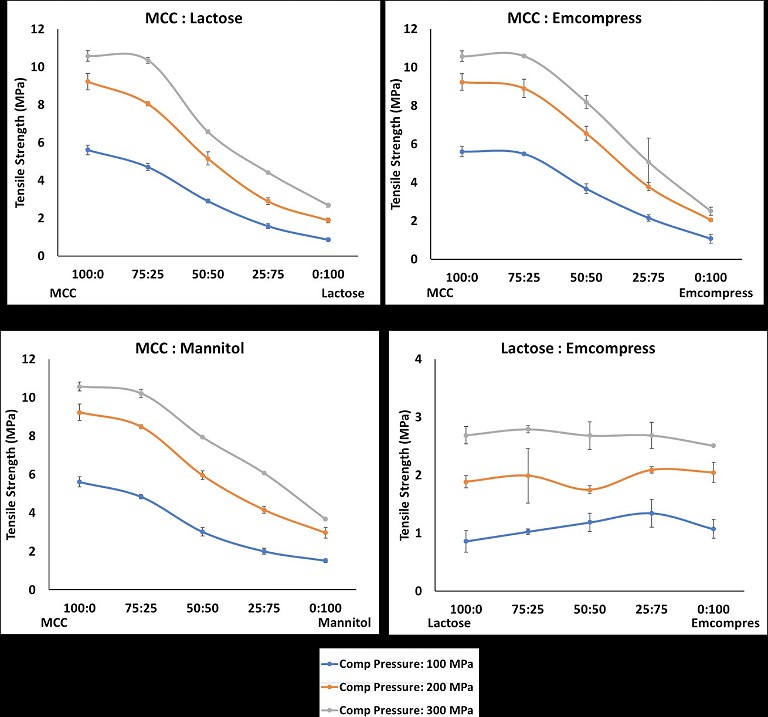
Figure 1: Change in the tablet mechanical strength of different binder-diluent proportions (A) MCC: α-lactose monohydrate; (B) MCC: Emcompress®; (C) MCC:Mannitol; and (D) α-lactose monohydrate: Emcompress®
Results and Discussion
An impact of mixing binder and diluent in different proportions is given in Figure 1. As expected, predominantly plastically deforming microcrystalline cellulose produced stronger tablets in the absence of other materials. The tablet mechanical strength of microcrystalline cellulose tablets decreased with addition of fragmenting (low and high) and brittle materials such as α-lactose monohydrates (Figure 1A),Emcompress® (Figure 1B), and mannitol (Figure 1C). It was interesting to observe that the addition of these materials in the microcrystalline cellulose led to a 16% to 85% reduction in the tablet mechanical strength of microcrystalline cellulose. These changes were the direct function of the material type and its amount. Addition of low fragmenting α-lactose monohydrates (Figure 1A) and high fragmenting Emcompress® (Figure 1B) reduced microcrystalline cellulose tablet mechanical strength from 2% to 85%, and 2% to 81%, respectively. This could be attributed to particle shielding of the predominantly plastic microcrystalline cellulose due to fragmentation of these materials in the early compression cycle. Such particle shielding could impair the plastic deformation of microcrystalline cellulose particles. This translates as a weakening of the particle bonding, and subsequent tablet mechanical strength reduction of even a very good binder like microcrystalline cellulose by 72%.5 Tablet weakening of plastic deforming materials in the presence of fragmenting and brittle materials could be compensated for by increasing the compression force. However, this reduction was compensated for between 88% to 213% and mainly dictated by the amount of fragmenting material. Having a relatively low impact of high fragmenting Emcompress® on microcrystalline cellulose tablet mechanical strength could be attributed to an increased number of bonding, due to extensive fragmentation, which is partially compensated by the reduced bonding due to impaired plastic deformation of microcrystalline cellulose.6 An addition of brittle mannitol reduced microcrystalline cellulose tablet mechanical strength by 3% to 73% (Figure 1C). A less negative impact of mannitol as compared to α-lactose monohydrates could be imparted to limited fragmentation and particle shielding effect of brittle mannitol.4,5
'This scale could serve as a ‘guiding torch’ for various formulators while designing their direct compression or dry granulation formulations'
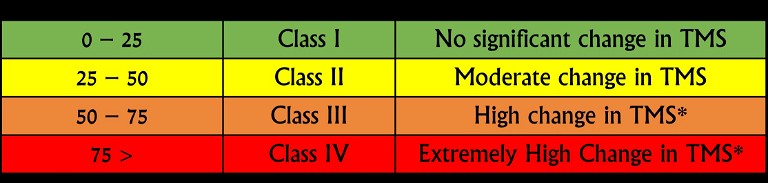
Figure 2: Newly proposed diluent-binder classification system demonstrating percent change in the tablet mechanical strength
'Tablet weakening of plastic deforming materials in the presence of fragmenting and brittle materials could be compensated for by increasing the compression force'
However, the synergistic effect of adding high fragmenting Emcompress® into low fragmenting α-lactose monohydrate tablet mechanical strength was observed (Figure 1D), specifically at 100 MPa compression pressure. Addition of Emcompress® into α-lactose monohydrate increased its tablet mechanical strength between 19% to 56% at 100 MPa compression pressure. This could be attributed to the increased number of bonds due to improve fragmentation of the resulting blend.6 However, a limited increase (0.37% to 11%) in the tablet mechanical strength of α-lactose monohydrate with addition of Emcompress® was observed when the compression pressure was 200 MPa and 300 MPa. It could be attributed to the increased fragmentation of α-lactose monohydrate at high compression pressure. This synergistic effect was further accelerated by 100% to 213% with an increase in the compression force. Clearly, addition of plastic, brittle and fragmenting (low and high) materials showed reduction or increase in the tablet mechanical strength in comparison to the single component systems.
Thus, a binder-diluent scale (Figure 2) is proposed based on this preliminary data. A change in the tablet mechanical strength was classified into four classes: Class I, II, III, and IV changes were defined as the reduction in tablet mechanical strength by 0-25%, 25-50%, 50-75% and 75-100%, respectively. A binder-diluent mixture exhibiting Class I and II changes could lead to insignificant to moderate change in the tablet mechanical strength. These changes could be less sensitive to CLPS issues. On the other hand, binder-diluent mixtures displaying Class III and IV changes might experience high to extremely high reduction in tablet mechanical strength. These mixtures will high have chances of CLPS issues. Current findings were further validated statistically with the least absolute shrinkage and selection operator (LASSO) model (Figure 3). This model exhibited neutral to positive coefficient (correlation) of mannitol, compression pressure and microcrystalline cellulose with tablet mechanical strength, while lactose exhibited a negative coefficient (correlation) with tablet mechanical strength.
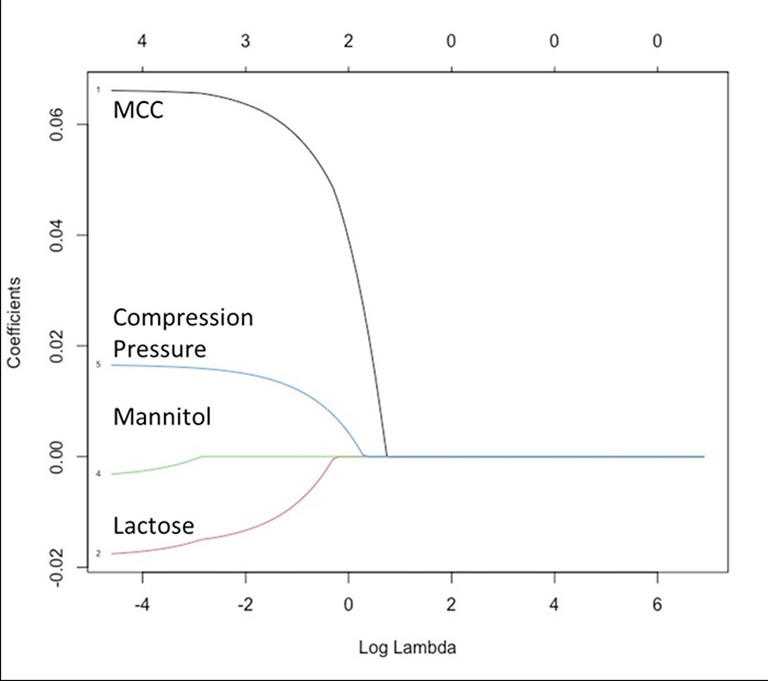
Figure 3: Least absolute shrinkage selection operator (LASSO) model indicating statistically significant positive impacts of microcrystalline cellulose (MCC) and compression pressure, neutral impact of mannitol and negative impact of α-lactose monohydrate (lactose) on tablet mechanical strength
Key Findings and Conclusions
Addition of brittle, low- and high-fragmenting diluents in the predominantly plastic binders such as microcrystalline cellulose exhibited Class III and Class IV change in its tablet mechanical strength as per the newly proposed classification system. Such changes could pose significant CLPS issues in the late development stage. These changes are a direct function of the binder-diluent ratio, which could be compensated for to some extent with an increase in the compression pressure. Interestingly, an addition of high fragmenting diluent with a low fragmenting diluent exhibited a synergistic or positive impact on the tablet mechanical strength of former ones. However, a binder-diluent mixture exhibiting a Class I and a Class II change in the tablet mechanical strength might produce tablets with a desired integrity. Certainly, these preliminary findings might serve as a ‘guiding torch’ while selecting the combination of various commonly used binders and diluents in the direct compression or dry granulation formulation development to the various formulators across the globe.
References
- Patel, S et al (2006), ‘Compression Physics in the Formulation Development of Tablets’, 23(1), 1-66
- Visit: fda.gov/regulatory-information/search- fda-guidance-documents/q8r2-pharmaceutical- development
- Haware R V et al (2009), ‘Application of multivariate methods to compression behavior evaluation of directly compressible materials’, European Journal of Pharmaceutics and Biopharmaceutics 72(1), 148-155
- Tarlier N et al (2018), ‘Deformation behavior of crystallized mannitol during compression using a rotary tablet press simulator’, Int J Pharm 547(1-2), 142-149
- Haware R V et al (2010), ‘Comparative evaluation of the powder and compression properties of various grades and brands of microcrystalline cellulose by multivariate methods’, Pharmaceutical Development and Technology 15(4), 394-404
- Alderborn G (1996), ‘Particle Dimensions’, Pharmaceutical Powder Compaction Technology 71, 245-282

Dr Devang Patel is a research scientist at Natoli Scientific. He is responsible for overseeing various research and formulation development projects while utilising his innovative approach. He also offers technical and scientific support to Natoli’s customers by conducting research on formulation and tablet tooling.

Kerry Cruz is the office manager and lab technician at Natoli Scientific. As a Lab Technician for Natoli Scientific, Kerry aids in the R&D of products for our customers by performing micromeritics, <1062> profiles, compression studies, and post compaction data. She also helps with blending, granulating, and tabletting of products that move to scale up batches and manufacturing.

Robert Sedlock is the director of Natoli Scientific and brings over 25 years of industry experience. In 2018 Robert opened the Natoli Scientific, Telford PA facility which is now a 14,000 square foot facility that offers solid dosage training programs, contract research and development projects from pre-formulation to formulation development and start-up manufacturing GMP services.

Dr Rahul Haware is a pharmaceutical scientist currently serving as the director of Scientific Support and New Business Development at Natoli Scientific. He is heading the ‘Preformulation’ wing of Natoli Scientific.